Kv4.2 subunit, a major contributor to transient K
+ outflow, is targeted by activity-dependent synaptic modulations and its functions are considerably mediated with the activities of synaptic glutamate receptors in CA1 neurons of rat hippocampi [
16,
23,
24]. These previous papers indicate that trafficking properties of Kv4.2 channels are rapidly driven by cellular signalings activated by synaptic activities. Because the rapid internalization of Kv4.2 channels by synaptic activities or plasticities resulted in the reduction of transient currents, the blockade of synaptic activities in CA1 neurons during recording was necessary for testing effects of HpTX
2 on K
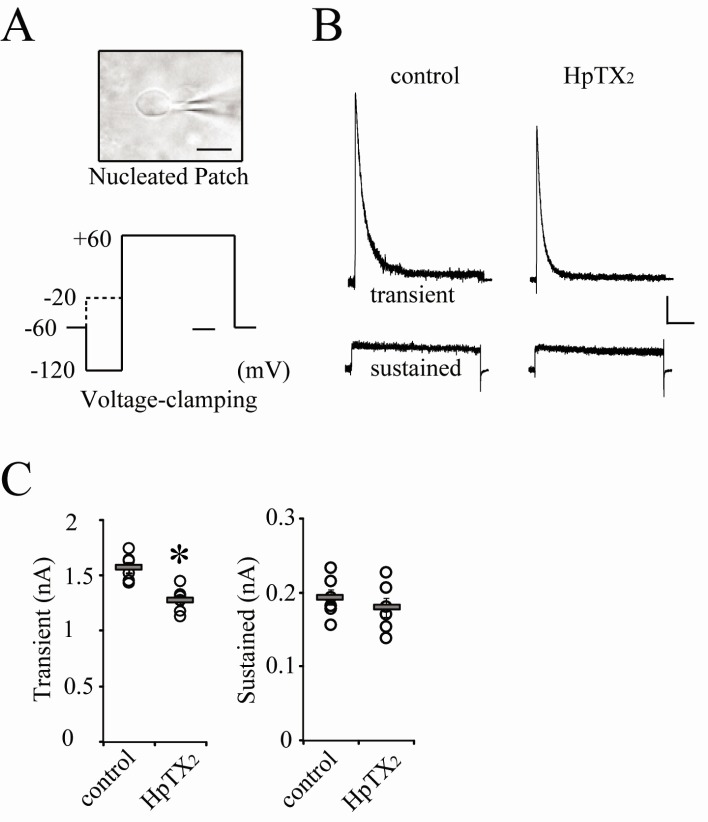
+ outward currents. For this, we used a nucleated-patch technique for isolating soma membrane of a CA1 neuron (
Fig. 1A). This methodological approach allowed us to test the intracellular or extracellular effects of HpTX
2 without influences of synaptic activities. In all experiments performed in this study, nucleated-patches were completely pulled within 2 min after making whole-cells, and neurons were rejected in cases of over 2 min. In the previous paper, the extracellular application of 100~200 nM HpTX
2 blocked only I
A currents through Kv4 subunits in rat ventricular myocytes [
18]. Therefore, we first tested 200 nM HpTX
2, which was added to external ACSF, to determine whether it could block transient currents in CA1 neurons. Total K
+ outward currents were recorded at +60 mV holding potential in a voltage-clamp mode, and sustained and transient currents were isolated by using a pre-pulse protocol (
Fig. 1A). Consistent with previous reports, the prominent effect of HpTX
2 was observed only on transient currents [
18,
19]. CA1 neurons showed a small but significant reduction of transient current amplitude in the presence of 200 nM HpTX
2, compared with neurons tested in normal recording solution (
Fig. 1B and C, transient, control=1.57±0.45, n=5; HpTX
2=1.32±0.71 nA, n=6; p<0.05). However, sustained currents were not affected by extracellular HpTX
2 (
Fig. 1B and C, sustained, control=0.19±0.01; HpTX
2=0.18±0.01 nA). These results indicate that existence of HpTX
2 in extracellular spaces can modulate the gating properties of Kv4.2 subunit in CA1 hippocampal neurons. Extracellular applications in 0.2 to 10 µM of HpTX
2 (
Supplementary Fig. 1) were also tested for confirming the concentration-dependent effects on CA1 neurons. All concentration of HpTX
2 significantly reduced transient currents of CA1 neurons, but no differences of reduction rates according to each concentration were observed. This indicates a restricted modulation of gating kinetics of I
A channels by HpTX
2 in CA1 neurons. Also, we confirmed that long exposure to HpTX
2 (2 µM, 24 hours) exhibited the similar rate of currents reduction, compared with short exposure effects. All concentrations of extracellular HpTX
2 showed no effects on sustained currents (
Supplemental Fig. 1).
Fig. 1
Extracellular HpTX2 reduces transient K+ outward currents in CA1 neurons. (A) The microscopic IR view of a nucleated-patch and a protocol of voltage-clamping to induce K+ outward currents. The scale bar inserted in the picture indicates 10 µm. Data acquired from experiments in which nucleated-patches were pulled within 2 min after making whole-cells, were used in this study. A pre-pulse (dotted line) to activate only sustained currents was applied for isolating transient currents from total outward currents. In all experiments, peaks of transient and sustained currents were generated at +60 mV holding potential. The time scale bar for voltage-clamping is 100 ms. (B) Example traces of transient and sustained currents recorded at +60 mV clamping in CA1 neurons. Currents were recorded at 10 min after pulling a nucleated-patch with (HpTX2) or without (control) HpTX2 in external solution. Scale bars indicate 0.2 nA and 100 ms. (C) All individual (circles) and averaged (square bars) amplitudes of transient (left panel) and sustained (right panel) currents are plotted for each group (control and HpTX2). Error bars represent standard error of mean (SEM). *: p<0.05, compared with control.

It is possible that blocking or modulating effects of toxins on gating properties of channels are variable regarding binding affinities between toxins and channels. In
Fig. 2, the next experiments were performed in order to determine if intracellular application of HpTX
2 also has effects to block only transient currents in neurons. Using internal pipette solution including either 100 or 200 nM HpTX
2, nucleated-patches were pulled within 2 min after making a whole-cell. Unexpectedly, both 100 and 200 nM of HpTX
2 were effective on sustained but not transient currents. Approximately 30% of the peak amplitude of sustained currents was reduced by intracellular HpTX
2 five minutes after making a whole cell (
Fig. 2, 200 nM HpTX
2; sustained, 0 min=0.19±0.08, 5 min=0.14±0.04, p<0.01; transient, 0 min=1.59±0.11, 5 min=1.52±0.12 nA; n=11). The reduction of sustained currents by HpTX
2 seemed not to be dose-dependent, showing a similar decrement between 100 (n=5) and 200 nM treatments (
Fig. 2B). Altering intracellular components is critical for determining channel permeability and neuronal responses, and cytosolic space of soma is significantly restricted under a nucleated-patch condition. Therefore, addition of HpTX
2 to an internal solution might possibly affect channel conductances without pharmacological actions. For clarifying this issue, we analyzed changes of current density, which was measured by whole-cell patch capacitances (
Fig. 2C). All neurons recorded with intracellular HpTX
2 (n=16) did not show significant changes of cell capacitances between 0 min (before recording, 19.8±3.2 pF) and 15 min (after recording, 21.1±2.2 pF) after pulling nucleated-patches, so it is clear that the densities of transient and sustained currents reflected the changes of peak amplitudes (
Fig. 2C, transient: 0 min=81.69±3.79, 15 min=74.81±4.02, p=0.248; sustained: 0 min=9.62±0.35, 15 min=6.54±0.29 pA/pF, p<0.01). In addition, we tested other agents known as blockers of K
+ channels to determine if they also have intracellular effects on either transient or sustained currents. 4-AP is widely used for suppressing K
+ outward currents in dendrites and soma, and was previously confirmed to be effective on transient currents when it was added to internal pipette solution [
13,
16]. In this study, 3 mM of 4-AP was added to internal solution, and nucleated-patches were then made (
Fig. 3A). 4-AP application resulted in a rapid and significant reduction of the peak amplitude in transient currents but not in sustained currents (
Fig. 3A, normalized amplitude at 10 min compared with 0 min; transient=0.35±0.04, p<0.05; sustained=0.86±0.02, n=5). We also tested Cs-gluconate internal solution (
Fig. 3B). In this experiment, both transient and sustained currents showed a gradual and significant reduction 10 min after pulling nucleated-patches (
Fig. 3B, Normalized amplitude at 10 min; transient=0.67±0.04, p<0.05; sustained=0.71±0.03, p<0.05, n=5). Therefore, the results shown in
Fig. 2 and
3 suggest that blocking effects of agents tested in this study may possibly be variable according to the location of channel effective sites [
18,
19]. In
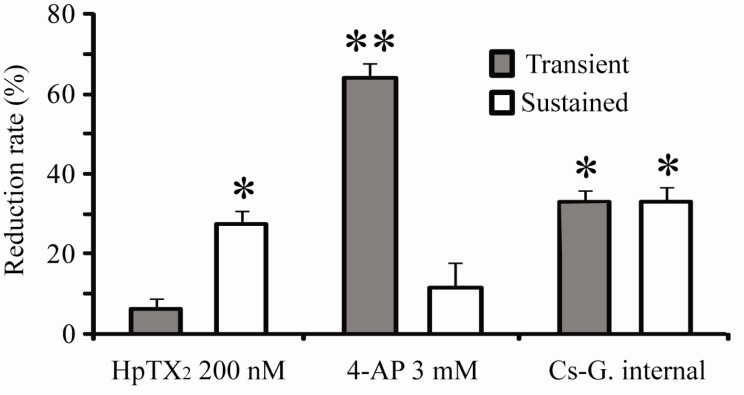
Fig. 4, the reduction rates of transient and sustained currents by intracellular drug treatments, which were calculated by the ratio between 0 min and 15 min peak after pulling nucleated-patches, were compared. The reduction rate of HpTX
2 in sustained currents was significantly larger than that in transient currents, while 4-AP reduced only transient currents (
Fig. 4, HpTX
2, transient:sustained=6.43±2.55: 27.68±3.22%; 4-AP, transient:sustained=64.15±3.51:18.02±6.08%). The reduction rates of Cs-gluconate internal sol. were statistically significant in both transient and sustained currents, (
Fig. 4, Cs-G. internal, transient-sustained=33.03±3.67:33.29±3.59%). These results suggest the possibility that K
+ outward flows in CA1 neurons can be selectively blocked by chemicals or toxins according to their application methods.
Fig. 2
Cytosolic HpTX2 reduces sustained but not transient K+ outward currents in CA1 neurons. (A) Example traces of both currents recorded immediately (0), 5, 10 and 15 min after pulling nucleated-patches. HpTX2 (200 nM) was added to internal pipette solution and normal external solution was perfused during recording. Scale bars indicate 0.2 nA and 100 ms. (B) Averaged peak amplitudes of transient (left panel) and sustained currents (right panel) recorded with intracellular 100 (gray circles) or 200 nM (dark circles) HpTX2. (C) Averaged current densities of transient and sustained currents measured at 0 min and 15 min after making nucleated-patches. The changes of current densities were acquired from data shown in (B) (100 nM: n=5, 200 nM: n=11). To calculate current density, the whole cell capacitance of 0 min was checked before recording currents, and that of 15 min was checked again right after completing the recording. Error bars represent SEM. **: p<0.01, compared with 0 min.
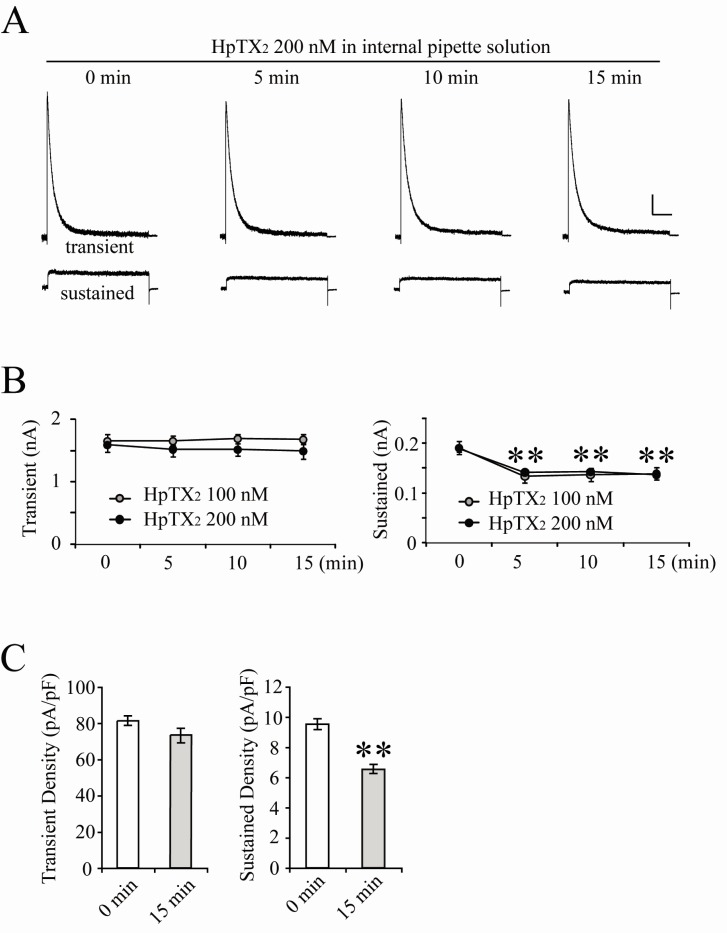

Fig. 3
Blocking effects of cytosolic 4-AP and Cs-gluconate internal solution on K+ outward currents. (A) Normalized individual and averaged values of peak amplitudes of transient and sustained currents recorded with 4-AP (3 mM) in internal pipette solution. (B) Normalized individual and averaged values of peak amplitude of transient and sustained currents recorded with Cs-gluconate internal solution. Error bars represent SEM. *: p<0.05 or **: p<0.01, compared with 0 min.
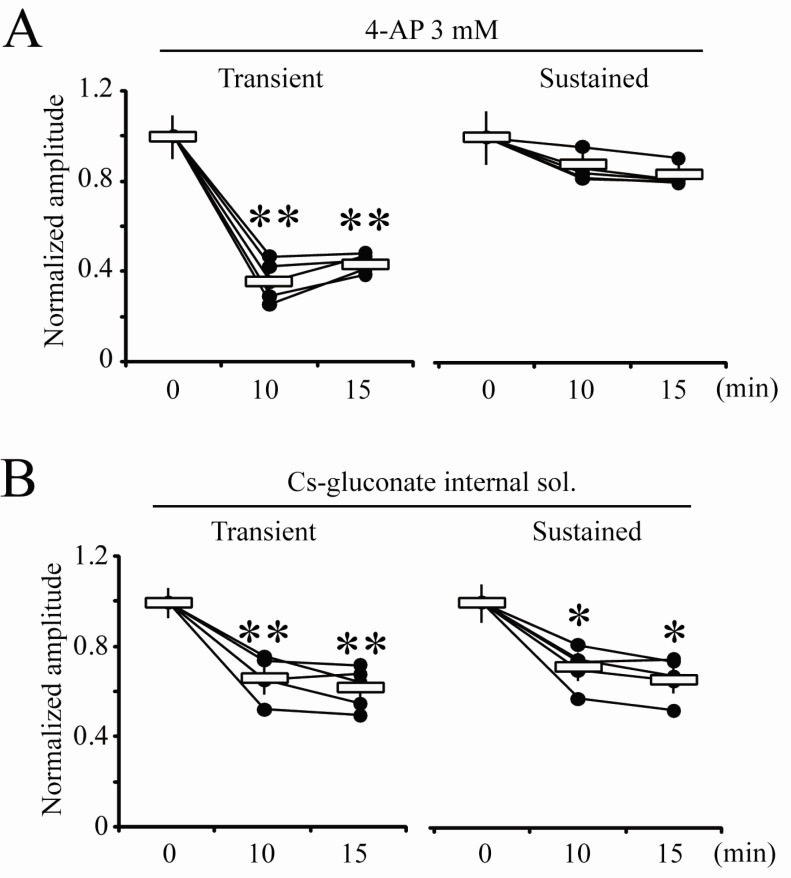

Fig. 4
Comparison of blocking effects on transient (gray bar) and sustained (open bar) currents induced by cytosolic drugs in hippocampal CA1 neurons. The reduction rate was calculated by the ratio of decreased amplitudes at 15 min after pulling nucleated-patches, compared with "0 min" peak amplitudes. Error bars represent SEM.
*: p<0.05 or
**: p<0.01, compared with "0 min" values shown in
Fig. 2 and
3.