INTRODUCTION
METHODS
Reagents
Solutions
Cell preparation
Recording of ionic currents
Data analysis and statistical test
RESULTS
Effect of sertraline on hERG, IKs, and IK1 currents
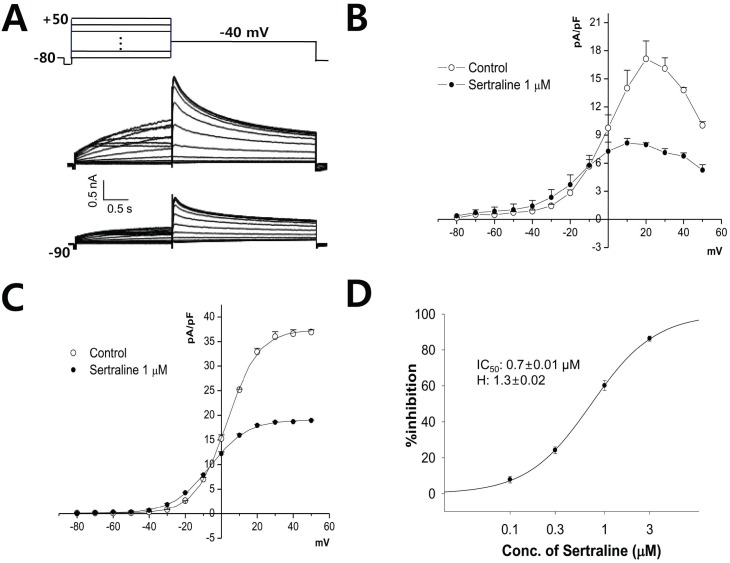 | Fig. 2The effect of sertraline on human ether-a-go-go-related gene (hERG) currents expressed in HEK293 cells. (A) Current responses to step voltage pulses of -80 to +50 mV in 10 mV steps from a holding potential of -80 mV (upper panel). Center: absence of sertraline, control condition; lower: in the presence of 1 µM sertraline. (B) Voltage-relationship of the hERG current measured at the end of depolarizing pulses against the pulse potential in the control and 1 µM sertraline. (C) Voltage-relationship of the tail current measured at its peak just after repolarization in control and after application of 1 µM sertraline. Data were fitted using Boltzmann equation. (D) Dose-response relationship for inhibition of hERG currents by serial application of 0.1, 0.3, 1, and 3 µM sertraline (n=4). The relationship was fitted to a Hill equation. The IC50 and Hill coefficient were 0.7 µM and 1.3, respectively. |
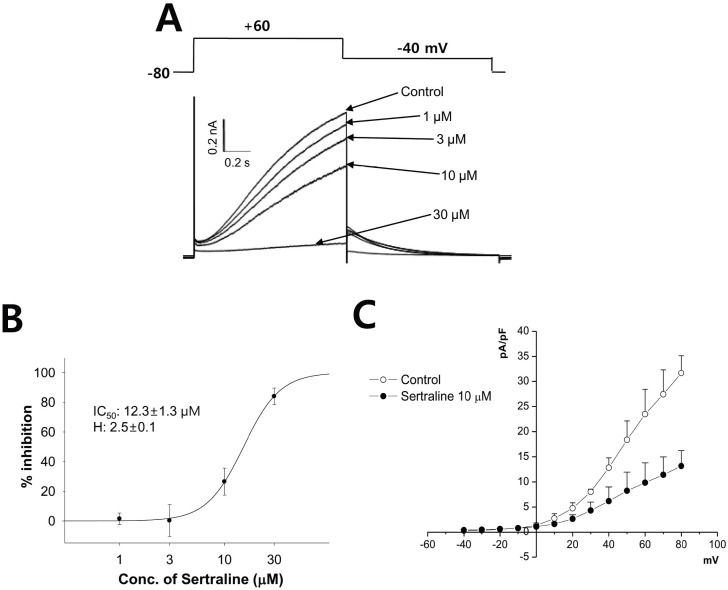 | Fig. 3The effect of sertraline on IKs expressed in HEK293 cells. (A) The cells were depolarized to +60 mV from a holding potential of -80 mV, followed by a 3-s repolarization back to -40 mV (upper). Representative current traces under control condition and after application of 1, 3, 10, and 30 µM sertraline (lower). (B) Concentration response curve for inhibition of IKs by serial application of 1, 3, 10, and 30 µM sertraline. The relationship was fitted to a Hill equation. The IC50 and Hill coefficient were 12.3 µM and 2.5, respectively (n=4). (C) Voltage-relationship of the peak IKs measured at the end of depolarizing pulses against the pulse potential in the control and 10 µM sertraline (n=4). |
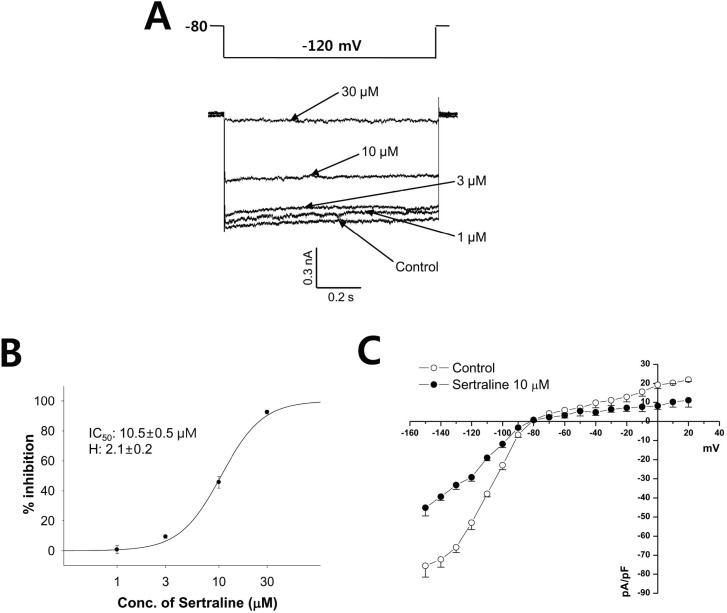 | Fig. 4The effect of sertraline on IK1 expressed in HEK293 cells. (A) The IK1 was elicited by the voltage of a one-step pulse (lasting 1 s) from -80 mV to -120 mV (upper). Representative current traces under control condition and after application of 1, 3, 10, and 30 µM sertraline (lower). (B) Concentration response curve for inhibition of IK1 by serial application of 1, 3, 10, and 30 µM sertraline. The relationship was fitted to a Hill equation. The IC50 and Hill coefficient were 10.5 µM and 2.1, respectively (n=4). (C) Voltage-relationship of the IK1 measured at the end of hyperpolarizing pulses against the pulse potential in the control and 10 µM sertraline (n=4). |
Effect of sertraline on INa
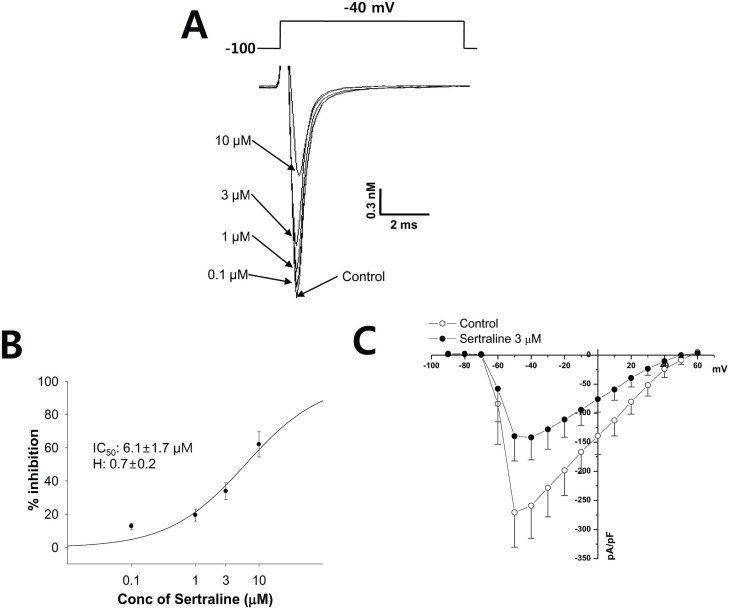 | Fig. 5The effect of sertraline on INa expressed in HEK293 cells. (A) The peak inward INa was generated by pulses of 20 ms duration to -40 mV from a holding potential of -100 mV delivered at a frequency of 10 Hz (upper). Representative current traces under control condition and after application of 0.1, 1, 3, and 10 µM sertraline (lower). (B) Concentration response curve for inhibition of INa by serial application of 0.1, 1, 3, and 10 µM sertraline. The relationship was fitted to a Hill equation. The IC50 and Hill coefficient were 6.1 µM and 0.7, respectively (n=4). (C) Voltage-relationship of the INa measured at its peak just after depolarization pulse against the pulse potential in the control and 3 µM sertraline. |
Effect of sertraline on ICa
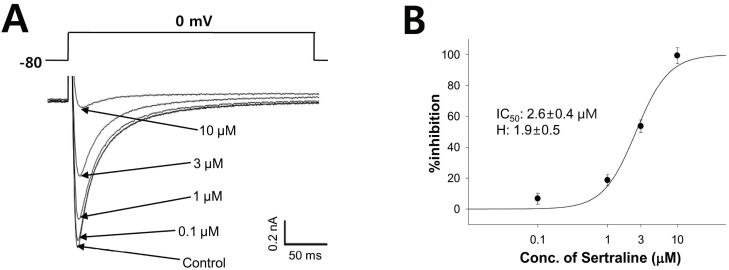 | Fig. 6The effect of sertraline on ICa in rat ventricular myocytes. (A) The peak of the ICa was induced by a single 500 ms voltage pulse to 0 mV from the holding potential of -80 mV (upper). Representative current traces under control condition and after application of 0.1, 1, 3, and 10 µM sertraline (lower). (B) Concentration response curve for inhibition of ICa by serial application of 0.1, 1, 3, and 10 µM sertraline. The relationship was fitted to a Hill equation. The IC50 and Hill coefficient were 2.6 µM and 1.9, respectively (n=4). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download