Abstract
Resveratrol, a natural compound, has been shown to possess anti-cancer, anti-aging, anti-inflammatory, anti-microbial, and neuroprotective activities. In this study, we examined the antiproliferative and cytotoxicity properties of resveratrol in Rat B103 neuroblastoma cells; although it's molecular mechanisms for the biological effects are not fully defined. Here, we examined the cellular cytotoxicity of resveratrol by cell viability assay, antiproliferation by BrdU assay, DNA fragmentation by DNA ladder assay, activation of caspases and Bcl-2 family proteins were detected by western blot analyses. The results of our investigation suggest that resveratrol increased cellular cytotoxicity of Rat B103 neuroblastoma cells in a dose-and time-dependent manner with IC50 of 17.86 µM at 48 h. On the other hand, incubation of neuroblastoma cells with resveratrol resulted in S-phase cell cycle arrests which dose-dependently and significantly reduced BrdU positive cells through the downregulation of cyclin D1 protein. In addition, resveratrol dose-dependently and significantly downregulated the expression of anti-apoptotic protein includes Bcl-2, Bcl-xL and Mcl-1 and also activates cleavage caspase-9 and-3 via the downregulation of procaspase-9 and -3 in a dose-dependent manner which indicates that involvement of intrinsic mitochondria-mediated apoptotic pathway. In conclusion, resveratrol increases cellular cytotoxicity and inhibits the proliferation of B103 neuroblastoma cells by inducing mitochondria-mediated intrinsic caspase dependent pathway which suggests this natural compound could be used as therapeutic purposes for neuroblastoma malignancies.
Neuroblastoma, one of the few human malignancies known to demonstrate spontaneous regression from an undifferentiated state to a completely benign cellular appearance originating from the sympathetic nervous system, is the most frequently occurring extra-cranial malignancy in childhood [1,2]. It is a disease exhibiting extreme heterogeneity, and is stratified into three risk categories: low, intermediate, and high risk. Low-risk disease is most common in infants and good outcomes are common with observation only or surgery, whereas high-risk disease is difficult to treat successfully even with the most intensive multi-modal therapies available [2]. However, despite many advances in diagnosis and standard interventions in the past three decades, neuroblastoma has remained a formidable challenge to clinical and basic scientists [3]. Therefore, the need exists to develop novel agents to improve treatment outcomes in this high-risk group. Strikingly, we would search for novel therapeutic strategies for neuroblastoma; natural product has attracted interest to achieve new approach for chemotherapy.
To address these questions, we have here reassessed resveratrol, a natural phytoalexin chemically known as 3, 4', 5-trihydroxystilbene, is a polyphenolic antioxidant present in red wine, grapes, berries, peanuts, etc. [4]. However, proposed benefits of resveratrol include neuroprotection, as well as cancer suppression is well studied [5-7]. The cancer chemopreventive potential of resveratrol is related to activation of the mitochondrial pathway of apoptosis, which are strongly implicated in cancer progression [8-11]. In addition to cardioprotective effects, resveratrol has also been found to exhibit anti-cancer properties, as suggested by its ability to suppress proliferation of a wide variety of tumor cells, including leukemia and cancers of the breast, lung, stomach, colon, liver, pancreas, and prostate [12].
The precise molecular mechanism(s) of resveratrol-induced apoptosis in Rat B103 neuroblastoma cells by which resveratrol exerts its anticancer effect has not yet been elucidated. A new dimension in the management of neoplasia was the increasing awareness that chemoprevention, which refers to the administration of chemical agents to prevent carcinogenesis [13]. A large number of chemopreventive and chemotherapeutic agents have been discovered from natural products and these provide a promising strategy to fight against cancer by inducing apoptosis in malignant cells [14,15]. Resveratrol had been shown to exhibit cancer chemopreventive effects in different systems based on its striking inhibition of a variety of diverse cellular events associated with tumor initiation, promotion and progression [8]. The antitumor activity of resveratrol has been indicated by its apoptosis-inducing ability in numerous cell types [8,16,17]. Resveratrol induces apoptosis in human leukemia cells as demonstrates by DNA fragmentation, an increased proportion of sub-diploids in the cell population, and a dose-dependent increase in cleavage of the caspase substrate poly (ADP-ribose) polymerase (PARP) [16]. Caspase activation occurs via cascade signaling events, in which the activation of initiator caspases would lead to the activation of downstream executive caspases [18-20] and ultimately triggers the apoptotic cell death program [21]. In the present study, we investigated the effects of resveratrol on cellular viability, cell cycle arrest, and apoptosis in Rat B103 neuroblastoma cell line. However, treatment of neuroblastoma cells with resveratrol was found to anti-proliferative effects which markedly induce S phase cell cycle arrest via the downregulation of cyclin D1 protein. Interestingly, resveratrol also was found to induce cytotoxicity of B103 cells in a dose- and time-dependent manner as mediated through the downregulation of mitochondrial anti-apoptotic proteins and the activation of intrinsic caspase mediated apoptosis in B103 neuroblastoma cells.
Resveratrol was purchased from Sigma-Aldrich, Chemical Co., (St. Louis, MO). The chemical were dissolved in dimethyl sulfoxide (DMSO) at a stock solution of 10 mM and then it was diluted with medium to obtain the working concentration. Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco/BRL (Grand Island, NY). Antibodies against caspase-3, cyclin D1, Mcl-1, Bcl-2, and Bcl-xL were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Beta-actin was obtained from Cell Signaling Technology (Beverly, MA). All other reagents were of analytical grade or of the highest purity available.
Rat B103 neuroblastoma cells were grown at 37℃ under a humidified atmosphere of 5% CO2. The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) plus 10% fetal bovine serum, 50 U/ml penicillin and 50 µg/ml streptomycin. Cells were starved with DMEM containing 0.5% FBS. After 24 h starvation, the cells were treated with various concentrations of resveratrol as described in the figure legends.
Cell viability was determined using a cytotoxity assay kit, the CCK-8 (Dojindo Lab, Japan) according to the manufacturer's protocol. The cells were plated into 96-wells to a density of 50~60% confluence. After 24 h incubation in starvation media, the cells were treated with various concentrations of resveratrol. After treatment of 48 h, the CCK-8 (10 µl) was added to each wells of the plates and incubated the plate for 3 h. A 96-well microtitre plate reader (Molecular Devices) was used to determine the absorbance at 450 nm for the CCK-8. The mean concentrations in each set of three wells were measured.
The cells were plated into 24-wells plates at 37℃ under a humidified atmosphere of 5% CO2. After 24 h when the density was 50~60% confluence than starvation media was added. After 24 h starvation, the cells were treated with various concentrations of Resveratrol. For the cell morphology experiment, the culture plates were examined under a Bright-Field Microscope and photographed.
For detection of apoptotic DNA cleavage, the DNA fragmentation assay was performed using ladder DNA fragmentation assay. In brief, cells were collected after treatment at a various concentrations of resveratrol as described in the figure legends and washed in PBS. The cells were then lysed with 500 µl of genomic DNA extraction buffer (0.1 M Nacl, 10 mM EDTA, 0.3 M Tris-HCl, 0.2 M sucrose, pH 8.0). The lysate was incubated with 20 µl of 10% SDS solution and incubated at 65℃ for 30 min. Added 120 µl potassium acetate (pH 5.3) and stored on ice for 1 h after that centrifuged for 10 min at 4℃ 12,000 rpm. Added 2 µl (10 mg/ml) RNase to supernatant, and incubated for 30 min at room temperature. The DNA was extracted by washing the resultant pellet in phenol/chloroform extraction and precipitaion by ethanol and then dissolved pellet with distilled water. DNA fragmentation was visualized by electrophoresis in a 0.8% agarose gel containing ethidium bromide.
B103 cells were grown on a round cover slides in 24 well dishes until the cells reached 80% confluence. The cells were treated with 0 and 15 µM resveratrol for 48 h and then pulsed with 10 µM BrdU (5-bromo-2'-deoxyuridine) for last 2 h. After fixing with 4% paraformaldehyde for 20 min, cells were permeabilized with 0.5% Triton X-100 for 15 min. The nuclei of cells were denaturated with 2 N HCl for 10 min, washed three times with 0.1 M sodium borate (pH 8.5). Following incubation with 10% normal serum to block nonspecific binding for 1 h at room temperature and cells were incubated overnight at 4℃ with anti-BrdU antibody diluted in PBS solution. Then, the cells were washed four times with PBS and incubated secondary antibody for 2 h at room temperature after that washed three times with PBS and stained using an ABC kit (Vector) for 2 h at room temperature and apply 0.05% diaminobenzidine tetrahydrochloride (DAB) to observe colour change until 3~4 min. The cells were mounted by polyvinyl alcohol and photographed at 400× magnification with a microscopy. The numbers of labeled cells were counted.
B103 cells were starved on 60 mm culture dishes in DMEM with 0.5% FBS for 24 h. Cells were pretreated with various concentration of resveratrol as indicated in each figure legend and then washed twice with ice-cold PBS. Cells were lysed in lysis buffer (2% SDS, Na3VO4 and protease inhibitor cocktail). After incubation on ice for 10 min sonicated 10 sec in 10% amplitude, the lysates were centrifuged (13,000 g, 20 min). Supernatants were collected and protein concentrations were determined by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of protein were separated by SDS-PAGE (8% to 15% reducing gels), transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), and blocked with 5% non-fat milk. Membranes were incubated in primary antibody overnight at 4℃. Membranes were then washed in TBST (10 mM Tris, 140 mM NaCl, 0.1% Tween-20, pH 7.6), incubated with appropriate secondary antibody, and washed again in TBST. Bands were visualized by enhanced chemiluminescence (ECL) and exposed to X-ray film.
To characterize the effect of resveratrol on neuronal cell viability, Rat B103 neuroblastoma cells were treated with various concentrations of resveratrol for 48 h, and viability was assessed by cell viability assay. We used 0 to 20 µM of resveratrol to determine its effect on neuronal cell viability. With the increasing resveratrol concentrations, the percentage of cell viability was decreased after 48 h incubation. The IC50 determined after 48 h incubation was 17.86 µM. After 5 µM concentration, resveratrol dose dependently decreased cellular viability, while at a concentration of 10 to 20 µM the number of viable cells was significantly decreased as compared by control (Fig. 1A). Further, a time exposure result was decreased in the viable number of B103 cells. The viability decreased significantly in response to 15 µM of resveratrol at 24 h incubation. When the experimental time period was increased upto 72 h, at same concentration, it has shown a significantly large number of cell deaths (Fig. 1B). Taken together these data demonstrated that resveratrol increased cellular cytotoxicity in a dose- and time-dependent manner.
To observe the morphological effects of B103 cell at 48 h incubation of resveratrol at 15 µM resveratrol was examined under a Bright Field Microscope and photographed. It showed that damaged cells which had become round, neurite retraction, membrane blebbing and shrunken at 15 µM of resveratrol, while the untreated B103 cells were well spread (Fig. 1C). To further confirm their morphological effects, we examined internucleosomal DNA fragmentation, which occurs during apoptosis, was assessed using a DNA gel electrophoresis. No DNA fragment was found in untreated B103 cells but DNA fragments were observed in cells treated with 15 and 20 µM resveratrol after 48 h, indicating that the cells underwent apoptosis (Fig. 1D). These results indicate that the morphological changes of B103 cell by resveratrol was due to apoptosis which shown fragmented DNA.
Immunocytochemistry was carried out to check whether resveratrol can affect the synthesis of DNA in cells using a thymine analog BrdU which gets incorporated during DNA synthesis by the bromodeoxyuridine (BrdU) incorporation assay. Resveratrol decreased the number of nuclei undergoing DNA synthesis. At 15 µM concentration, most of the cells synthesizing DNA were eliminated as compared to control (Fig. 2A). DNA synthesis evidences by BrdU incorporation showed that the percentage of cells in the S-phase decreased from 28.18 positive cells in control samples to 15.07 positive cells after resveratrol treatment at 15 µM (Fig. 2B). The result clearly revealed that treatment of B103 cells with resveratrol may affect DNA synthesis levels and caused a reduction in the BrdU positive cells (brown stained). We, therefore, checked that the reduction in cyclin D1 levels to resveratrol-induced G1 phase arrest. Indeed, resveratrol treatment of B103 neuroblastoma cancer cells potently reduced the most important cell cycle protein cyclin D1 (Fig. 2C). Consistent with the established function of cyclin D in promoting cell cycle progression through G1 to S-phase, the reduction in cyclin D1 levels by resveratrol led to a dramatic reduction of cell growth. As measured by densitometry analysis cyclin D1 protein was reduced near 0.2 fold at 5 µM concentrations and after 10 to 20 µM resveratrol significantly and dose-dependently decreaed the expression of cyclin D1 protein (Fig. 2D). Collectively, this S-phase arrest suggests that the anti-proliferative effect of resveratrol during apoptosis is mediated at least in part by the downregulation of cyclin D1.
To understand the molecular mechanisms involved in the activation of apoptosis induced by resveratrol, we first evaluated whether this natural compound might regulate anti-apoptotic Bcl-2 family proteins expression in B103 cells treated with resveratrol (0 to 20 µM) for 48 h. Western blotting and densitometry analysis demonstrated that treatment of B103 cells with resveratrol resulted in a dose-dependently reduction in the levels of the anti-apoptotic proteins Bcl-2, Bcl-xL and Mcl-1 compared with untreated cells (Fig. 3A). As measured by densitometry analysis Bcl-2 protein was reduced 0.1 fold at 5 µM concentrations and after 10 to 20 µM resveratrol significantly and dose-dependently decreased the expression of Bcl-2 protein (Fig. 2B). Concomitantly, levels of Bcl-xL and Mcl-1 proteins were significantly and dose-dependently reduced after 5 to 20 µM of resveratrol (Fig. 2C, D). This observation suggestes that resveratrol treatment can alter the protein levels of key members of the Bcl-2 family, which may contribute to the susceptibility of cancer cells to mitochondrial dysfunction.
To elucidate the molecular events required to involve in death by the activation of apoptosis induced by resveratrol in B103 cells, we next assessed the levels of some caspases including caspase-9 and -3 by western blot and densitometry analysis. However, we found that active form of caspase-9 and caspase-3 were dose-dependently up-regulated induced by resveratrol in B103 cell, but remarkably, procaspase-9 and -3 was downreguled at 48 h incubation (Fig. 4A). As shown by densitometry analysis, resveratrol dose-dependently upregulated cleaved caspase-9 expression in which at least 3.9 fold increases at 5 µM concentrations and after 10 to 20 µM resveratrol significantly and dose-dependently increased (Fig. 4B), while cleaved caspase-3 also was dose-dependently and significantly up-regulated during this apoptosis process in B103 cells compare to untreated cells (Fig. 4C). To ensure this effect was a direct activation of the effector caspase-3 activation we used caspase-3 inhibitor in resveratrol-induced B103 cells. Interestingly, we found that resveratrol-induced cellular apoptosis was almost significantly decreased by the caspase-3 inhibitor, DEVD-FMK, in B103 cells (Fig. 4D). Therefore, it is clear that resveratrol selectively induces apoptosis through the intrinsic caspase mediated pathway in B103 neuroblastoma cells.
The present study was designed to define the mechanism(s) of the cellular antiproliferative and cytotoxic properties of resveratrol in Rat B103 neuroblastoma cell which suggesting to development of a novel approaches for the management of cancer cells. However, there are no reports regarding the mechanisms involved in the induction of programmed cell death by resveratrol in B103 cells. In this study, we also investigated the molecular mechanism of apoptosis in B103 cells and focused our mechanistic investigations on caspases activation as well as Bcl-2 family protein expression. Additionally, it will also be necessary to determine why the degree of response to resveratrol varies among different types of molecular mechanisms in neuroblastoma cancer cells.
Apoptosis is one of the most prevalent pathways through which chemotherapeutic agents can inhibit the overall growth of cancer cells [8,16]. In vitro as well as in vivo studies have suggested that resveratrol may impart chemopreventive effects against certain cancers including skin cancer [22,23]. Our main finding was that half-maximal inhibition of cell viability was observed in the above 17 µM range of resveratrol, and IC50 values decreased with increasing incubation doses and times (Fig. 1). However, concentrations that caused cytotoxic effects of cell morphology and induced apoptosis detected by DNA fragments were observed to be higher than the untreated cells (Fig. 1). This might be due to an initial inhibition of DNA synthesis and arrest of cells in the S-phase (Fig. 2A, B) through the dose-dependently and significantly downregulation of cyclin D1 protein expression (Fig. 2). This observation suggests that the potential in vitro efficacy of resveratrol in B103 cells to be useful in the prevention of neuroblastoma carcinoma. Moreover, it remains to be determined whether resveratrol suppresses the development of neuroblastoma cancer in both animal and human cancer models.
Bcl-2 family proteins play an important role in the regulation of the mitochondrial pathway, since these proteins localize to intracellular membranes such as the mitochondrial membrane [24]. They comprise both anti-apoptotic members, e.g. Bcl-2 or Bcl-xL, as well as pro-apoptotic molecules such as Bax and BH3 domain only molecules, e.g. Bid [24]. Imbalances in the ratio of anti- and pro-apoptotic Bcl-2 proteins may favour tumor cell survival instead of cell death [24]. In addition, altered expression of Bcl-2 family proteins has been reported in various human cancers [25]. In this study shown that treatment of B103 cells with resveratrol resulted in a dose-dependently and significantly decreased in the levels of the anti-apoptotic proteins Bcl-2, Bcl-xL and Mcl-1 (Fig. 3). The finding of this study demonstrates that the alteration in Bcl-2 family proteins contributed to an increase in mitochondrial membrane permeability in resveratrol-treated Rat B103 neuroblastoma cells via the intrinsic-mediated apoptotic pathway.
In the mitochondria-mediated pathway, apoptotic stimuli enhances the permeability of the outer mitochondrial membranes and releases pro-apoptotic factors into the cytoplasm which subsequently bind to apoptosis protease-activating factor 1 (Apaf-1) and inactive procaspase-9 to form an apoptosome, thereby resulting in caspase-9 activation which triggers the subsequent cleavage of caspases-3. Because most cell types require activation of the caspase pathway if apoptosis is occur that might activate caspase-9 and caspase-3, a key executioner of apoptosis [26-28], whose activation is essential to apoptosis [29,30]. Moreover, activation of caspase-3 results in cleavage of cellular substrates critical for cell survival, such as PARP which cleaved DNA [29,30]. In the present study, we found that resveratrol dose-dependently and significantly activated caspase-3 via the downregulation of procaspase-9 and-3 (Fig. 4) as a results cell become apoptotic death. Based on our observations, we suggest that resveratrol-induces apoptosis are mediated through the mitochondria-mediated intrinsic caspase pathway.
In summary our studies have demonstrates that resveratrol treatment of Rat B103 neuroblastoma cells resulted in an S-phase cell cycle arrest which dounregulates cyclin D1 protein and underwent apoptosis via the process of DNA fragmentation. In addition, resveratrol downregulates the expression of anti-apoptotic Bcl-2 family proteins and activates caspase which suggests that involvement of intrinsic mitochondria-mediated apoptosis in B103 cells. Therefore, the present study suggests that resveratrol would be used as a potential therapeutic purpose in neuron cancer cell for the patients with neuroblastoma malignancies.
Figures and Tables
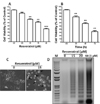 | Fig. 1Resveratrol increases cellular cytotoxicity of Rat B103 neuroblastoma cells. (A, B) B103 cells were cultured in 96-well culture dishes to near confluence 50~60% and then starved in DMEM containing 0.5% FBS for 24 h. Cell death was determined by using the cytotoxicity assay kit (CCK-8, Dojindo Lab). Cells were exposed to resveratrol in a different dose of 0 to 20 µM in dose dependent and 15 µM in time dependent experiments. Each point is mean±SEM of quintuple samples. Data are mean from three independent experiments in which the activity in the absence of resveratrol versus in the presence of resveratrol is significantly different (n=3, **p<0.01). (C) B103 cells were grown in 24-well culture dishes to near confluence 50% and then starved in DMEM containing 0.5% FBS for 24 h. They were then added 15 µM concentration of resveratrol and grown at 37℃, in humidified 5% CO2 for 48 h and then morphology was observed by Bright-Field Microscopy. Arrows indicate cells with apoptotic morphology. (D) B103 cells were grown in 100 mm culture dishes to near confluence 90% and then starved in DMEM containing 0.5% FBS for 24 h. The cells were then treated with 0, 15 and 20 µM of resveratrol. After 48 h resveratrol treatment, DNA was extracted and separated on 0.8% agarose gel containing ethidium bromide. DNA fragments were visualized under UV light. M indicates as a Marker. GA (Gambogic acid) used as a positive control. |
 | Fig. 2Antiproliferative effects resveratrol in DNA synthesis and cyclin D1 downregulation. (A) B103 cells were culture on a round cover slides in 24 well dishes until 80% confluence in 10% FBS. The cells were then treated in the absence and presence of 15 µM of resveratrol. After 46 h resveratrol treatment, cells were incubated with 10 µM BrdU for 2 h before fixation and subsequent immunostaining with an anti-BrdU antibody (Arrow shows BrdU positive cells). (B) The percentages of stained cells were counted in three independent random areas. Results are means±SE and representatives of three independent experiments are shown (n=3, *p<0.05). (C) B103 cells were cultured in 60-mm culture dishes to near 80% confluence in 10% FBS. They were treated with 0 to 20 µM of resveratrol for 48 h. Equal volumes of whole-cell extracts containing 40 µg of protein were separated and electrophoretically blotted. Cyclin D1 was detected via immunoblot analysis. β-actin was used as a loading control. (D) The intensities of the cyclin D1 bands were determined by densitometric scanning and analyzed by Bio-Profil software and the expression levels were normalized to β-actin. Results are mean±S.E. and representatives of three independent experiments are shown (n=3, *p<0.05, **p<0.01). |
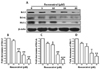 | Fig. 3Resveratrol down-regulates Bcl-2 family proteins. (A) B103 cells were cultured in 60-mm culture dishes to near 90% confluence and then starved in DMEM containing 0.5% FBS for 24 h. After 24 h starvation cells were treated with 0 to 20 µM of resveratrol. Whole cell lysates were subjected to 15% SDS-PAGE and the levels of Bcl-2, Bcl-xL, and Mcl-1 were detected by western blotting as described in materials and methods. β-actin was used as a loading control. (B~D) The intensities of the Bcl-2 bands, the Bcl-xL bands and the Mcl-1 bands were determined by densitometric scanning and analyzed by Bio-Profil software and the expression levels were normalized to β-actin. Results are mean±S.E. and representatives of three independent experiments are shown (n=3, *p<0.05, **p<0.01, ***p<0.001). |
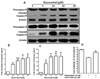 | Fig. 4Effects of resveratrol in caspase expression. (A) B103 cells were cultured in 60-mm culture dishes to near 90% confluence and then starved in DMEM containing 0.5% FBS for 24 h. They were treated with 0 to 20 µM of resveratrol for 48 h. Levels of caspase-9 and -3 proteins were detected by western blotting. β-actin was used as a loading control. (B, C) The intensities of the cleaved caspase-9 bands and the cleaved caspase-3 bands were determined by densitometric scanning and analyzed by Bio-Profil software and the expression levels were normalized to β-actin. Results are mean±S.E. and representatives of three independent experiments are shown (n=3, *p<0.05, **p<0.01). (D) B103 cells were cultured in 96-well dishes to near confluence 50~60% and then starved in DMEM containing 0.5% FBS for 24 h. After starvation cells were pretreated with 10 µM DEVD-FMK for 1 h just before exposure to 15 µM resveratrol for 48 h. Cell number was quantified by CCK-8 kit. Results are means±SE and representatives of three independent experiments are shown (n=3, *p<0.05). |
ACKNOWLEDGEMENTS
This research was supported by Hallym University Research Fund (HRF-201209-028), Basic Science Research Program through the National Research Foundation of Korea (NRF, 2012-0003013) and Priority Research Centers Program through NRF funded by the Ministry of Education, Science and Technology (NRF, 2012R1A6A1048184), the Republic of Korea.
References
1. Bénard J, Raguénez G, Kauffmann A, Valent A, Ripoche H, Joulin V, Job B, Danglot G, Cantais S, Robert T, Terrier-Lacombe MJ, Chassevent A, Koscielny S, Fischer M, Berthold F, Lipinski M, Tursz T, Dessen P, Lazar V, Valteau-Couanet D. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol Oncol. 2008. 2:261–271.
2. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007. 369:2106–2120.
3. Castel V, Grau E, Noguera R, Martínez F. Molecular biology of neuroblastoma. Clin Transl Oncol. 2007. 9:478–483.
4. Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009. 53:115–128.
5. Roccaro AM, Leleu X, Sacco A, Moreau AS, Hatjiharissi E, Jia X, Xu L, Ciccarelli B, Patterson CJ, Ngo HT, Russo D, Vacca A, Dammacco F, Anderson KC, Ghobrial IM, Treon SP. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenström's macroglobulinemia. Clin Cancer Res. 2008. 14:1849–1858.
6. Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009. 6:70–81.
7. Pallàs M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A. Modulation of sirtuins: new targets for antiageing. Recent Pat CNS Drug Discov. 2008. 3:61–69.
8. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997. 275:218–220.
9. Pozo-Guisado E, Lorenzo-Benayas MJ, Fernández-Salguero PM. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int J Cancer. 2004. 109:167–173.
10. Komina O, Wesierska-Gadek J. Action of resveratrol alone or in combination with roscovitine, a CDK inhibitor, on cell cycle progression in human HL-60 leukemia cells. Biochem Pharmacol. 2008. 76:1554–1562.
11. Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm Res. 2009. 26:211–217.
12. Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004. 24:2783–2840.
13. Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997. 278:1073–1077.
14. Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000. 130:2S Suppl. 467S–471S.
15. Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000. 21:525–530.
16. Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998. 92:996–1002.
17. Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem. 1997. 30:91–113.
18. Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004. 16:139–144.
19. Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004. 23:2950–2966.
20. Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999. 274:20049–20052.
21. Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005. 115:2665–2672.
22. Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999. 428:305–327.
23. Carbó N, Costelli P, Baccino FM, López-Soriano FJ, Argilés JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999. 254:739–743.
24. Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000. 256:50–57.
25. Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999. 17:2941–2953.
26. Bertoncello I, Bradley TR, Watt SM. An improved negative immunomagnetic selection strategy for the purification of primitive hemopoietic cells from normal bone marrow. Exp Hematol. 1991. 19:95–100.
27. Buick RN, Till JE, McCulloch EA. Colony assay for proliferative blast cells circulating in myeloblastic leukaemia. Lancet. 1977. 1:862–863.
28. Minden MD, Buick RN, McCulloch EA. Separation of blast cell and T-lymphocyte progenitors in the blood of patients with acute myeloblastic leukemia. Blood. 1979. 54:186–195.
29. Datta R, Banach D, Kojima H, Talanian RV, Alnemri ES, Wong WW, Kufe DW. Activation of the CPP32 protease in apoptosis induced by 1-beta-D-arabinofuranosylcytosine and other DNAdamaging agents. Blood. 1996. 88:1936–1943.
30. Ibrado AM, Huang Y, Fang G, Liu L, Bhalla K. Overexpression of Bcl-2 or Bcl-xL inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res. 1996. 56:4743–4748.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download