Abstract
We analyzed the pharmacokinetics of C3G on data from twelve subjects, after 2-week multiple dosing of black bean (Phaseolus vulgaris, Cheongjakong-3-ho) seed coat extract, using the mixed effect analysis method (NONMEM, Ver. 6.2), as well as the conventional non-compartmental method. We also examined the safety and tolerability. The PK analysis used plasma concentrations of the C3G on day 1 and 14. There was no observed accumulation of C3G after 2-week multiple dosing of black bean seed coat extract. The typical point estimates of PK were CL (clearance)=3,420 l/h, V (volume)=7,280 L, Ka (absorption constant)=9.94 h-1, ALAG (lag time)=0.217 h. The black bean seed coat extract was well tolerated and there were no serious adverse events. In this study, we confirmed that a significant amount of C3G was absorbed in human after given the black bean seed coat extract.
Antioxidants are molecules capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizing agent. Oxidation reactions can produce free radicals and these radicals can start chain reactions. When the chain reaction occurs in a cell, it can cause cell damage or death. Antioxidants may terminate these chain reactions by removing free radical intermediates, and inhibit other oxidation reactions [1].
Hence, plants and animals maintain complex systems of multiple types of antioxidants. Either low levels of antioxidants or inhibition of antioxidant enzymes may cause oxidative stress and may damage or kill cells. As oxidative stress appears to be an important initiator of many human diseases, the use of antioxidants has been intensively studied, particularly as treatments for stroke and neurodegenerative diseases [2,3]. Antioxidants have also been widely used as dietary supplements and have been investigated for their potential in the prevention of diseases, such as cancer and ischemic heart disease [4].
In the black bean seed coat, there are functional ingredients, such as anthocyanins, which are known as powerful antioxidants (0.87~23.52 mg/g), as well as dietary fibers (80%). The black pigmentation is due to accumulation of anthocyanins in the epidermis palisade layer of the seed coat and cyanidin-3-glucoside (C3G, 80.9% of total content) is the most abundant anthocyanine [5].
There are some reports on the antioxidative effect of anthocyanins extracted from the black soybean seed coat [2-4]. However, only limited information is available on the anthocyanin's kinetics when the black beans were given to humans. In this study, we analyzed the pharmacokinetics (PK) of C3G from black bean using the mixed effect analysis method, as well as the conventional non-compartmental method. The safety and tolerability of black bean seed coat extract, when given 1 g per day orally for 2 weeks, have also been examined.
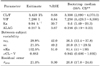
Healthy volunteers, aged 20 to 45 years, with no clinically relevant conditions identified based on medical history, physical examination, laboratory tests and electrocardiography (ECG), were eligible for inclusion. Subjects with any history that could result in alteration of C3G metabolism or with hypersensitivity to beans were excluded. Finally, 12 subjects were enrolled (Table 1).
An open-label, randomized, multiple dosing clinical study was conducted at a single center (Clinical Trial Center, Seoul St. Mary's Hospital). All subjects were given 1 g of black bean seed coat extract every morning for 14 days. The trial was designed and monitored in accordance with the good clinical practice guidelines of Korea and with the principles of the Declaration of Helsinki. The independent institutional review board (Seoul St. Mary's Hospital) approved the protocol before execution of the trial, and all participants gave written informed consent.
Subjects were admitted to the Seoul St. Mary's Hospital clinical trial center on the evening of day -1 and day 13. During the outpatient period, subjects were required to maintain low-phenolic diet and to report daily to the clinical trial center for dosing and diet. They were dosed at approximately 9 AM, prior to breakfast, and were instructed to fast for at least 10 hours prior to dosing on days 1 and 14, and for at least 4 hours thereafter.
On the morning of days 1 and 14, subjects had 7 ml of peripheral venous blood sampled predose and at 0.25, 0.5, 1, 1.5, 2, 4 and 6 hours after dosing. Blood was collected in heparin tubes placed on ice, and then centrifuged at 3000rpm for 10 minutes at 4℃. The separated plasma was stored at -70℃ until assayed for anthocyanins.
Twenty kg of black beans (Cheongjakong-3-ho) havested in 2010 was used to extract anthocyanin at a certified GMP unit. The bean seed coat was filtered and extracted for 24 hours with 80% ethanol. The extract was finally packaged into 7,400 capsules containing 200 mg extract in each capsule.
Plasma concentration of C3G was determined by a validated method using high-performance liquid chromatography coupled with tandem mass spectrometry (API4000, AB SCIEX, Foster City, CA, USA). C3G and malvidin-3-galactoside (M3G), as the internal standard, were purchased from Extrasynthese (Genay, France).
Plasma samples were acidified immediately with 6 M HCl of 1/20 volume after centrifugation at 3,000 rpm for 10 min at 4℃ and frozen at -70℃ until analysis. For analysis, plasma samples (500 µl) were prepared by diluting 250 µl 0.1% formic acid, after spiking 25 µl internal standard at 800 ng/ml. HLB cartridges (30 mg, 1 cc, Waters) were previously conditioned with 1 ml MeOH and 1 ml 0.1% formic acid; then, samples were loaded and cartridges were washed with 1 ml 0.1% formic acid. Finally, the analytes were eluted with 500 µl 80% ACN with 0.1% formic acid. The fractions were evaporated after collection and the residue was reconstituted in 200 µl of 10% MeOH with 0.1% formic acid, and 10 µl was injected into a LC/MS/MS system [6].
In the HPLC system, Luna C18 column (5 µm, 100×2.0 mm, Phenomenex) was used with a gradient mobile phase consisting of 0.1% formic acid:methanol with 0.1% formic acid (80:20 to 20:80, v/v) and a flow rate of 500 µl/min. The MS/MS system was operated in the positive ionization mode with electrospray and multiple reaction monitoring mode. Transition ions at m/z 449.18 to 287.30 and 493.16 to 331.04 were monitored for C3G and M3G, respectively.
The lower limit of quantification for C3G was 0.2 ng/ml, with calibration curves ranging from 0.2 to 50 ng/ml. The precision (relative standard deviation) and mean intra- and inter-day accuracies were ≤10.00% and 99.36 to 100.7%, respectively. These results demonstrated that the plasma concentration analysis was reliable over the given range.
Initially, the pharmacokinetic analysis of C3G was performed using non-compartmental analysis (NCA) (Phoenix WinNonLin 6.2; Pharsight, Mountain View, CA, USA). The actual blood sampling times were used in the pharmacokinetic analysis. The maximum concentration (Cmax) of drug in serum at Day 1 and Day 14, and the time to reach Cmax (Tmax) were obtained from the observed values. The area under the plasma-concentration-time curve until the last observation (AUClast) was estimated using the log-linear trapezoidal rule. The terminal half life was calculated by dividing 0.693 by λz.
Statistical analysis was carried out using R software Version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to describe the PK parameters of C3G and the paired t-test was used to evaluate the changes in PK parameters on day 1 and day 14.
The concentration-time data for C3G were analyzed using NONMEM version 6.2 (Icon Development Solutions, Ellicott City, MD, USA) with the G77 FORTRAN compiler. The first-order conditional estimation (FOCE) method with interaction was used throughout the model building.
One- and two-compartment open models with first-order elimination were tested to estimate the clearance (CL), central volume of distribution (V1), peripheral volume of distribution (V2), intercompartmental clearance (Q), absorption constant (Ka) and lag time (ALAG), using the ADVAN subroutines. Models were selected based upon a decrease in the objective function value (OFV) of more than 3.84 (p-value 0.05 in approximate χ2 distribution) and improvement in individual plots, as well as scatterplots. A log normal distribution was assumed for inter-individual variability (η), and the PK parameters of the jth subject (Pj) were therefore described using the following equation:
Where TVP represents the typical population value of PK parameters, such as CL, V, Ka and ALAG. The inter-individual variability eta (η) for each PK parameter was assumed to follow a Gaussian distribution with a mean 0 and a variance ω2. Possible correlations between the inter-individual variabilities for CL and V were also estimated. The inter-occasion variabilities (IOV) for CL, V, and Ka, i.e. the variability within one individual between study days, was also examined with the exponential random effects term:
Where Pjk is the jth individual parameter value at the day (occasion) of k that differs from the typical individual value by an additional random effect κjk·ηj·κjk and were assumed to be symmetrically distributed with a mean of 0 and a variance of ω2.
As for the residual error, the additive, proportional and combined forms were tested. An example of the combined error form is shown, as follows:
Where IPREDij is the individual predicted concentration and Yij is the measured concentration of the jth individual at the ith sampling time, and εij is residual errors. Residual errors (ε) include intra-individual variability, assay error and model misspecification. They were also assumed to follow a Gaussian distribution with a mean 0 and variance σ2.
Age, height, weight, sex and creatine clearance were screened as potential covariates of the parameters using Generalized Additive Modeling (GAM) implemented in Xpose version 4.0.4.
In forward selection of covariates, variables that decreased the minimized OFV greater than 3.84 (p<0.05) and decreased the omega value were selected. Covariates that did not increase the minimized OFV more than 3.84 (p<0.05) in backward elimination were removed from the model.
Ninety-five percent confidence intervals (CIs) for mean population PK parameters were determined using the re-sampling technique based on the bootstrap method. One thousand re-sampled datasets were estimated using the population PK model. The model was also evaluated by visual predictive checks (VPC) with 1,000 simulated datasets.
Paired t-tests were used to evaluate the changes in PK parameters on day 1 and day 14. The results are shown in Table 2.
There were no significant changes in AUClast and Cmax on Day 1 and Day 14. No accumulation of C3G after 2-week multiple dosing of black bean seed coat extract was found.
A one-compartment first-order elimination model with combined residual errors was chosen as the basic model. After establishment of the basic model, the inter-occasional variability between day 1 and day 14 was tested for all PK parameters, but was finally found relevant only for absorption rate constant (Ka). Thereafter, the effects of covariates on relevant PK parameters were explored. There was no covariate that decreased the OFV and inter-individual variability. The final model was as follows.
Where Ka is absorption rate constant and Ke is terminal elimination constant.
Where Kaj represents absorption rate constant of the jth individual, TVKa is typical value of the absorption rate constant and Kjk is the interoccasional variability of jth individual.
Ninety-five percent confidence intervals (CIs) for mean population PK parameters were determined using the re-sampling technique based on the bootstrap method. One thousand re-sampled datasets were estimated using the final population PK model. The 2.5th and 97.5th percentiles of the mean population PK parameters were regarded as the lower and upper 95% CI limits, respectively (Table 1).
VPCs were performed by using simulated concentrations of 1,000 virtual datasets (nsub=1,000 in the $SIMULATION block, 1,000 virtual patients) from the final model. The result from VPC showed that the PK model gave acceptable predictive performance. Curves for the 5th, 50th and 95th percentiles of concentrations were overlaid on the observed concentrations on day 1 and day 14 (Fig. 2).
Overall, black bean seed coat extract was well tolerated. All 12 subjects completed the study.
There were no serious adverse events (AEs) or discontinuations due to AEs during the entire study period, but only one subject reported one minor adverse event, i.e. acute tonsilitis, which was mild and unlikely related to the consumption of black bean seed coat extract. The review of the ECG data did not reveal any safety concerns. There were no clinically relevant changes in terms of physical examinations, ECGs, vital signs or laboratory tests, including clinical biochemistry, hematology and urinalysis.
In this study, we evaluated the pharmacokinetics, as well as safety and tolerability, of C3G after 2-week administration of black bean seed coat extract. To predict the exposure to C3G in healthy subjects population, simulation based upon the mixed effect analysis method was also used. There were no severe adverse events during the entire study period but only one minor adverse event, which was unlikely to be related to the consumption of black bean.
Our data demonstrate that anthocyanins, especially C3G, are present in sufficient quantities in plasma for the PK parameters to be determined after oral intake of 1 g of the extract. The elimination of anthocyanins in plasma appeared to follow first-order kinetics, consistent with results from other studies [7,8].
In this study, the mean half-life of C3G (calculated with the CL and Vd estimated from the model) was 1.5 h, which was more than one hour shorter than that of other studies [8,9]. The differences in the formulations used in the studies may have caused this difference. In our study, we used black bean seed coat extract, which was highly soluble and the absorption rate was very fast when given fasted; in contrast, in other studies, subjects ate the raw fruit pulp, powder or other extract [8,9]. With its short half-life in human plasma, there was no observed accumulation of C3G after 2-week multiple dosing in our study.
In terms of the AUC of C3G, black bean seed coat extract 1g was equivalent to about 250 ml of acai pulp and to about 30 g of black raspberry powder [8,9].
As well as C3G itself, its metabolites produced in the body or bowel and other anthocyanins in the black bean seed coat may also have antioxidative effects. Therefore, further studies are needed to compare the antioxidative effects of these natural products.
Figures and Tables
Fig. 1
Goodness-of-fit plots for the final population PK model of C3G. Black line, line of identity; gray line, LOESS (locally weighted regression smooth line). IWRES, individual weighted residuals.

ACKNOWLEDGEMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ007186), Rural Development Administration, Republic of Korea. We thank Ms. Hyo-Bum Seo and Yeon-Hee Lee for their excellent services in C3G analysis.
References
1. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997. 82:291–295.
2. Kelly PJ, Morrow JD, Ning M, Koroshetz W, Lo EH, Terry E, Milne GL, Hubbard J, Lee H, Stevenson E, Lederer M, Furie KL. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008. 39:100–104.
3. Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996. 27:1124–1129.
4. Gey KF, Brubacher GB, Stähelin HB. Plasma levels of antioxidant vitamins in relation to ischemic heart disease and cancer. Am J Clin Nutr. 1987. 45:5 Suppl. 1368–1377.
5. Kim SL, Kim HB, Chi HY, Park NK, Son JR, Yun HT, Kim SJ. Variation of anthocyanins and isoflavones between yellow cotyledon and green cotyledon seeds of black soybean. Food Sci Biotechnol. 2005. 14:778–782.
6. Ling Y, Ren C, Mallery SR, Ugalde CM, Pei P, Saradhi UV, Stoner GD, Chan KK, Liu Z. A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J Chromatogr B Analyt Technol Biomed Life Sci. 2009. 877:4027–4034.
7. Cao G, Muccitelli HU, Sánchez-moreno C, Prior RL. Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study. Am J Clin Nutr. 2001. 73:920–926.
8. Mertens-talcott SU, Rios J, Jilma-stohlawetz P, Pacheco-palencia LA, Meibohm B, Talcott ST, Derendorf H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008. 56:7796–7802.
9. Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, Singh A, Sanders J, Aziz R, Casto B, Sun X. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005. 45:1153–1164.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download