Abstract
The hypothalamus-pituitary-adrenocortex (HPA) axis is the central mediator of the stress response. The supramammillary (SuM) region is relatively unique among the hypothalamic structures in that it sends a large, direct projection to the hippocampal formation. It has been shown that mild stress could activate the SuM cells that project to the hippocampus. However, the role of these cell populations in modulating the stress response is not known. The present study examined the effect of stress on different populations of SuM cells that project to the hippocampus by injecting the fluorescent retrograde tracer, fluorogold (FG), into the hippocampus and utilizing the immunohistochemistry of choline acetyltransferase (ChAT), corticotrophin releasing factor (CRF), serotonin (5-HT), glutamate decarboxylase (GAD), tyrosine hydroxylase (TH) and NADPH-d reactivity. Immobilization (IMO) stress (2 hr) produced an increase in the expression of ChAT-immunoreactivity, and tended to increase in CRF, 5-HT, GAD, TH-immunoreactivity and nitric oxide (NO)-reactivity in the SuM cells. Fifty-three percent of 5-HT, 31% of ChAT and 56% of CRF cells were double stained with retrograde cells from the hippocampus. By contrast, a few retrogradely labeled cells projecting to the hippocampus were immunoreactive for dopamine, γ-aminobutyric acid (GABA) and NO. These results suggest that the SuM region contains distinct cell populations that differentially respond to stress. In addition, the findings suggest that serotonergic, cholinergic and corticotropin releasing cells projecting to the hippocampus within the SuM nucleus may play an important role in modulating stress-related behaviors.
The hypothalamus-pituitary-adrenocortex (HPA) axis is the central mediator of stress response. This neuroendocrine axis functions to coordinate neural, endocrine and immune responses to diverse stressful stimuli that threaten homeostasis [1]. The supramammillary (SuM) nucleus of the hypothalamus is thought to serve as an 'interface' relaying inputs to hypothalamic and limbic structures involved in the control of behavioral functions [2]. The SuM nucleus lies dorsal to the mammillary bodies in the caudal diencephalon and has strong connections with the limbic systems. In particular, the SuM region sends a large, direct, projection to the hippocampal formation [3,4]. SuM fibers distributing to the hippocampus mainly originate from the lateral two-thirds of SuM region [5] and predominantly innervates two regions of the hippocampal formation, the dentate gyrus throughout the hippocampus and the CA2/CA3 region of Ammon's horn of the dorsal hippocampus. Most of SuM neurons projecting, via collaterals, to the hippocampal CA2/CA3 were predominantly located in the lateral part of SuM region around the mammillothalamic tract [6]. Previous studies have shown that SuM cells contain a variety of neurotransmitters and neuropeptides including acetylcholine (Ach), dopamine, serotonin, GABA, aspartate, glutamate, and enkephalin [7-12].
The SuM area of the hypothalamus, although small, may have modulatory effects on hippocampal as well as related temporal cortex activities [9]. It has been shown that SuM cells have been implicated in generation of hippocampal theta activity, [13,14] the function of memory, anxiety and reward [15,16]. This nucleus is thought to play an important role in these functions through its inputs to the limbic systems, especially, the hippocampus [6,17]. The hippocampus is known to play a role in learning, memory and emotion; it is a major component of the neuroanatomical stress circuit. This structure is involved in terminating HPA axis responses to stress and attenuates stress responses by shutting off this axis [18].
Previous studies have shown that IMO stress upregulates expression of c-fos, one of immediate early genes, in several brain areas and the restraint stress-induced expression of c-fos in the hypothalamus was concentrated mainly in the SuM region [19,20]. It has been shown that placement in a novel environment results in activation of SuM cells projecting to the hippocampus [21], suggesting that the SuM region may contain several populations of neurons which are differentially responsive to certain behavioral manipulations. However, the role of these cell populations in modulating the stress response is not known.
The purpose of this study was to examine the effect of stress on different populations of SuM cells that project to the hippocampus, by a combination of immunohistochemistry of acetylcholine (ChAT), dopamine (TH), corticotrophin releasing factor (CRF), serotonin (5-HT), GABA (GAD67), and nitric oxide (NADPH-d), and retrograde tracing techniques following IMO stress.
Sprague-Dawley rats (Orient Animal Corp, Kyunggido, Korea), weighing 220~240 g each were used for the experiments. The male rats were group-housed (three per cage) under a reversed light-dark cycle (light on from 08:00~20:00 h). The room temperature was 20~25℃ and the humidity was 30±35%. The rats had free access to food and water. All the rats were handled daily for at least 1 week prior to the experiment.
The reagents used for the study were Hydroxystillbamidine methanesulfonate (Fluorogold; Molecular Probes Inc.) Choline acetyltransferase (ChAT; Sheep polyclonal antibody, Chemicon, Temecula, CA, USA), Corticotrophin releasing factor (CRF; Rabbit polyclonal antibody, Santa-Cruz Biotechnology, USA), Serotonin (5-HT; Rabbit polyclonal antibody, Chemicon, Temecula, CA, USA), Glutamate decarboxylase (GAD67; Mouse monoclonal antibody, Chemicon, Temecula, CA, USA), Tyrosine hydroxylase (TH; Mouse antibody, Zymed,San Francisco, CA, USA), FITC (Goat anti-Rabbit IgG, Zymed, South San Francisco, CA, USA), FITC (Rabbit anti-Mouse IgG, Zymed, South San Francisco, CA,USA), FITC (Rabbit anti-Sheep IgG, Zymed, South San Francisco, CA,USA), β-NADPH (β-Nicotineamide adenine dinucleotide phosphate; Sigma-Aldrich), NBT (Nitro blue tetrazolium; Sigma-Aldrich).
Fluorogold was used as retrograde axonal tracer, although orthograde axonal transport does occured. For retrograde transport, the survival times should be varied from 4 days to 14 days. Fluorogold was microinjected into all of the rats. The rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed in a stereotaxic apparatus. The skull was firmly placed in the apparatus, and the scalp was shaved and cleaned with betadine; an incision was made through the skin and muscle to expose the skull and the skin was then retracted. Four percent of fluorogold in the normal sterile saline (0.9%), 0.5µl total volume, was microinjected via a 1µl Hamilton syringe at a rate of 0.1µl/min into the CA3 region of the hippocampus at coordinates: AP -5.3 mm, ML -5 mm, DV -5.2 mm relative to the bregma (Tracer Technology, LumaFluor, New York, NY, USA). All used coordinates were from the atlas of Paxinos and Watson [22]. After infusion of the fluorogold, the needle was left in place for 10 min before it was slowly retracted. The animals were allowed seven recovery days following the surgery prior to carrying out the experiments.
The animals were randomly divided into two groups: one group (n=15) was exposed to immobilization stress and the other group (n=15) was not stressed. The rats in the IMO group were acutely stressed by immobilizing them in a prone position for 2 hours in a rodent restraint conical vinyl, which prevented all forward/backward and lateral movements. The rats in the control group were only placed inside of cages without any physical restraint during the stress treatment.
After the IMO stress experiment, all of the animals were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and then perfused through the ascending aorta with normal 100 ml of saline (0.9%), followed by 800 ml of 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS). The brain was removed, post-fixed overnight and cryoprotected in 20% sucrose in 0.1 M PBS at 4℃. The brains were cut by cryostatsectioning into 30 µm coronal sections at the level of the SuM area, and these slices were processed histochemically as free-floating sections.
Immunohistochemical visualization of choline acetyltransferase (ChAT; Sheep polyclonal antibody), corticotrophin releasing factor (CRF; Rabbit polyclonal antibody), Serotonin (5-HT; Rabbit polyclonal antibody), glutamate decarboxylase (GAD67; Mouse monoclonal antibody), and tyrosine hydroxylase (TH; Mouse polyclonal antibody) was performed on the free-floating sections using antibodies and immunefluorescent staining methods.
The brain sections were washed in PBS containing 0.3% Triton X-100. Each primary antibody was evaluated against the following specific antigens: ChAT (2,000:1), CRF (500:1), 5-HT (500:1), GAD67 (500:1), and TH (2,000:1). The primary antibodies were diluted with blocking solution and the tissues were incubated for 24 h at 4℃ with constant agitation. Secondary antibodies (FITC-conjugated) were obtained from Jackson/Immunoresearch (West Grove, PA, USA) and used at a dilution of 200:1 (in PBS) and incubated for 1 h at RT. The sections were mounted using Vector Shield for confocal microscopic evaluation. Following a pre-rinse in phosphate buffered saline (PBS), three times, the tissues were incubated β-Nicotine amide adenine dinucleotide phosphate 1 mg and Nitro Blue Tetrazolium 1 mg/PBS 1 ml for 30~45 min at 37℃ with constant agitation. For NADPH-d staining, following rinsing in phosphate buffered saline (PBS), the sections were mounted on slides, air-dried and coverslipped for microscopic evaluation.
In order to verify the locations of the injected sites, all sections of the brain were mounted on gelatin-coated microscope slides. The sections were then rehydrated in 90 and 75% ethanol and water 2 min each and treated with ceresyl violet solution for 2 min. Subsequently, the sections were placed in 0.1% acetic acid in 75% ethanol for 2 min, rinsed in water, dehydrated and finally coverslipped with xylene for microscopic evaluation.
The numbers of cells were measured by placing the grid on the SuM region at the level of 4.2 mm bregma according to the atlas of Paxinos and Watson [22]. The cells of the SuM region were counted at 200× magnification using a microscope rectangle grid measuring 500×500 microns. Cells within the examined area were counted on each of 3 sections per animal.
The mean number of choline acetyltransferase (ChAT) immunoreactive cells in the examined regions of the SuM was 90.6±6.7 for the normal group and 137.3±12.6 for the IMO stress group. The ChAT immunoreactive cells of the IMO stress group were increased at the SuM region, compared with those of the normal group (p<0.001). The number of ChAT immunoreactive cells that were double-labeled with retrogradely labeled cells in the SuM region was 15.5±1.6 for the normal group and 27.9±8.1 for the IMO stress group, respectively. Thirty one percent of the ChAT immunoreactive cells in the SuM region were double labeled with retrogradely labeled cells projecting to the hippocampus (Fig. 3 and 8).
CRF immunoreactive cells in the examined regions of the SuM region were 58.4±8.5 and 65.4±7.3 for the normal and the IMO stress group. The number of CRF immunoreactive cells that were colocalized with retrogradely labeled cells in the SuM region was 49.4±9.1 for the normal group and 42.6±3.1 for the IMO stress group, respectively. Fifty-six percent of the CRF immunoreactive cells were double stained with retrogradely labeled cells projecting to the hippocampus (Fig. 4 and 8).
The mean number of 5-HT immunoreactive cells in the SuM region was 55.2±9 for normal group and 64.29±6.2 for the IMO stress group. The number of double labeled 5-HT immunoreactive and retrogradely labeled cells was 32.6±7.9 and 31.14±3.8 for the normal and the IMO stress group. The 5-HT immunoreactive cells in the SuM region were similar for the normal and stress-induced groups. In addition, 53 percent of the 5-HT cells were double stained with retrogradely labeled cells projecting to the hippocampus (Fig. 5 and 8).
GAD67 immunoreactive cells observed in the SuM region were not colocalized with retrogradely labeled cells projecting to the hippocampus as seen in Fig. 6. TH immunoreactive cells and fibers were observed in the SuM region. However, they were not co-labeled with retrogradely labeled cells (Fig. 6). NADPH-d reactive cells were not colocalized with retrogradely labeled cells in the SuM region (Fig. 7).
The results of this study demonstrated that stress could activate different populations of SuM cells that project to the hippocampus by utilizing a combination of fluorescence retrograde tracing and the immunohistochemical detection of dopamine, GABA, NO, Ach, CRF, and serotonin.
The present study clearly showed that many of retrogradely labeled cells projecting to the hippocampus were colocalized with ChAT, CRF and 5-HT immunoreactive cells in the SuM region. By contrast, the GAD, TH and NADPH-d reactive cells were minimally co-labeled with retrograde labeled cells. These findings suggest that the ChAT, CRF and 5-HT in SuM cells may play an important role in the hippocampus during IMO stress. The results of the present study demonstrated that many cholinergic cells were distributed in the SuM region. A significant number of cholinergic cells were observed in the SuM region after IMO stress. About 31% of ChAT immunoreactivity was colocalized with the retrogradely labeled cells in the SuM region. These results suggest that a majority of cholinergic cells in the SuM region appear to influence the hippocampus during IMO stress.
The cholinergic system is known to be involved in the information processing related to hippocampal learning and memory, and the central cholinergic neurons are of putative relevance to major depression [23]. Learning and memory in mammals, including humans, is dependent on the neurotransmitter acetylcholine, in the basal forebrain and hippocampus [24]. It has been shown that cholinergic cells in the SuM region play a role in the baroreflex and potentiate the reflex rise in blood pressure to occlusion of the carotid artery, the so-called carotid arterial occlusion reflex [8]. Neurotransmitters such as Ach are known to produce behavioral, neuroendocrine, cardiovascular, and noradrenergic effects reflecting stress.
Therefore, the biosynthetic enzyme for Ach, ChAT, is currently the most reliable biomarker for cholinergic neuron activity [25]. The SuM region is involved in the control of hippocampal theta rhythms and modulates the synaptic excitation of hippocampal neurons [13,14,26]. Theta is a rhythmic, electrical field activity of the hippocampus with a well-established behavioral correlation [12]. The ChAT-positive cells from the SuM region may modulate hippocampus-dependent behaviors in response to acute stress.
CRF-immunoreactive cells in the SuM region were found to be colabeled with the fluorogold-labeled cells projecting to the hippocampus. About 56% of the CRF cells were double-labeled with retrograde labeled cells in the SuM region. The CRF-immunoreactive cells were significantly increased after stress. The paraventricular nucleus of the hypothalamus (PVN) serves as the origin of the final common pathway in the secretion of glucocorticoid hormones in response to stress. IMO stress is acknowledged to be involved in psychological and physical stress by altering behavioral and physiological responses [27,28]. IMO stress acutely initiates a transient increase in CRF expression in the PVN and produces increased plasma corticosterone in the present study (data not shown).
The majority of CRF-immunoreactive cells have been found to colocalize with c-fos immunoreactive neurons in the SuM nucleus. Prior results showed that CRF-immunoreactive neurons were observed in the mammillary body [29]. The hippocampus plays an important role in the negative feedback of the limbic-hypothalamic-pituitary adrenal (LHPA) axis [30,31]. Hippocampal function is significantly influenced by glucocorticoids [32,33]. By acute modulation of neuronal excitability, glucocorticoids affect hippocampus-dependent behavior, such as spatial memory. Chronic changes in glucocorticoid levels impair hippocampal function and morphology, which ultimately leads to cognitive impairment [34]. These results suggest that CRF-immunoreactive cells in the SuM region are activated by stress and these cells may mediate the stress response. Therefore, CRF-immunoreactive neurons in the SuM region may influence spatial and cognitive memory process during stress through its inputs to the hippocampus.
We observed that the 5-HT immunoreactive cells were increased in the SuM region after immobilization stress. About 53% of the 5-HT immunoreactive cells were double labeled with fluorogold reactive cells in the SuM region. Our data suggest that serotonin-containing cells in SuM region may also modulate hippocampus-dependent behaviors during stress. Serotonin (5-HT) is known to be involved in a variety of behavioral changes in the stress models. Prior studies have shown that the SuM and mammillothalamic (Smt) nucleus are located along the path of serotonin fibers originating in the raphe nuclei of the brain stem. These fibers enter the hypothalamus dorsal to the mammillary bodies where they traverse the SuM region, and pass rostrally in the medial part of the medial forebrain bundle, close to the Smt [9]. In the CNS, it is a modulatory neurotransmitter participating in the regulation of many important brain functions like thermoregulation, sleep, aggression, and eating. Disturbed serotonergic signaling is an important mechanism underlying stress-related psychopathological states as anxiety, depression and eating disorders.
GABAergic, dopaminergic and nitric oxide positive cells are related to the stress response. GABA has been implicated in the regulation of various aspects of the stress response. Both GABAA and GABAB receptors are abundantly expressed in hippocampus [35]. Dopaminergic activity has been reported to be increased in the striatum and prefrontal cortex of rats exposed to stress. In addition, NO, as a neurotransmitter/neuromodulator, plays a role, directly or indirectly, in the regulation of the CNS, endocrine and behavioral process involved the stress response. However, GABAergic, dopaminergic and nitric oxide positive cells were minimally labeled with fluorogold reactive cells projecting to the hippocampus. These results suggest that a variety of neurons in the SuM region are differentially responsive to the stress response. Further studies are clearly needed to examine the roles of these cell populations including cholinergic, CRF, serotonin, GAD, TH and NO containing cells in mediating the stress-related behaviors such as alterations of learning and memory, anxiety or reward systems affected by stress.
Following the injections of fluorogold into the hippocampus, most of retrogradely labeled cells were observed around mammillothalamic tract in the SuM region, consistent with the previous study [21]. It should be noted that SuM fibers projecting to the hippocampus mainly originate from the lateral two-thirds of SuM region and approximately 5~10% of SuM neurons project to the CA2/CA3 of the hippocampus [5]. Therefore, cell distributions of the SuM region from our data may reflect only 5 to10 percent among the total populations of the SuM cells. These results suggest that caution is needed in interpreting results obtained from the tracing methods. In addition, since the retrograde injections only include a small region, hippocampal CA3, distributions or roles of other populations of SuM cells projecting to the different areas of the hippocampal formation including several subregions such as the CA1 or dentate gyrus, in stress response, should be elaborated and characterized in the future.
In conclusion, stress activates serotonergic, cholinergic and corticotropin releasing cells in the SuM region that project to the hippocampus. 31% of ChAT, 53% of 5-HT and 56% of CRF cells were double stained with retrograde cells projecting to the hippocampus, whereas dopaminergic, GABAergic and nitric oxide positive cells were minimally double labeled. The SuM region contains cells with different characteristics related to IMO stress and these cells may directly influence the hippocampus. The results of this study clearly demonstrated that the SuM region of the brain contains distinct cell populations that are differentially responsive to stress. These findings suggest that serotonin, acetylcholine and corticotropin releasing factor in the SuM region play an important role in modulating stress-related behaviors.
Figures and Tables
 | Fig. 1Camera lucida drawing of injection site of the hippocampus (A). Marked spot is enlarged in (B); (100×). |
 | Fig. 2Schematic drawings of the region of the supramammillary complex (A) and low magnification photographs showing the distribution of the retrogradely labeled cells following injections of flourogold into the hippocampus (B). MT, mammillothalamic tract; PH, posterior nucleus; SMT, submammillothalmic nucleus; SuMM, supramammillary medial; SuML, supramammillary lateral; MnM, medial mammillary median; MM, medial mammillary nucleus, medial part; ML, medial mammillary nucleus, lateral part; LM, lateral mammillary nucleus; PH, posterior nucleus. Scale bar; 500µm. |
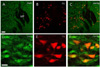 | Fig. 3Low (A~C) and high magnification (D~F) microphotographs showing the distribution of choline acetyltrasferase (ChAT)-like immunoreactive cells (A, D; in green) and retrogradely labeled cells (B, E; in red) after acute IMO stress. The overlay of images A+B, D+E is shown in C, F, respectively where yellow color illustrates co-localization of ChAT with retrogradely labeling. Scale bar in A, 100µm; D, 20µm. MT, mammillothalamic tract. |
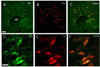 | Fig. 4Low (A~C) and high magnification (D~F) microphotographs showing the distribution of corticotropin releasing factor (CRF)-like immunoreactive cells (A, D; in green) and retrogradely labeled cells (B, E; in red) after acute IMO stress. The overlay of images A+B, D+E is shown in C, F, respectively where yellow color illustrates co-localization of CRF with retrogradely labeling. Scale bar in A, 100µm; D, 20µm. MT, mammillothalamic tract. |
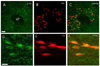 | Fig. 5Low (A~C) and high magnification (D~F) microphotographs showing the distribution of serotonin (5-HT)-like immunoreactive cells (A, D; in green) and retrogradely labeled cells (B, E; in red) after acute IMO stress. The overlay of images A+B, D+E is shown in C, F, respectively where yellow color illustrates colocalization of 5-HT with retrogradely labeling. Scale bar in A, 100µm; D, 20µm. MT, mammillothalamic tract. |
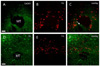 | Fig. 6Microphotographs showing the distribution of glutamate decarboxylase (GAD67) (A; in green) and tyrosine hydroxylase (TH) (D; in green) with retrogradely labeled cells (FG) (B, E; in red) after acute IMO stress. The overlay of images A+B and D+E is shown in C and F, respectively, where a few GAD67 and TH-like immuoreactive neurons were colocalized with retrogradely labeling (arrow). Scale bar in A and D; 100µm. MT, mammillothalamic tract. |
ACKNOWLEDGEMENTS
This work was supported by a KBSI grant (T31901) to SH and the Cognitive Neuroscience Program (M10644000017-06N4400-01710) of the Korea Ministry of Science and Technology and Republic of Korea.
References
1. Hesketh S, Jessop DS, Hogg S, Harbuz MS. Differential actions of acute and chronic citalopram on the rodent hypothalamic-pituitary-adrenal axis response to acute restraint stress. J Endocrinol. 2005. 185:373–382.
2. Gonzalo-Ruiz A, Morte L, Flecha JM, Sanz JM. Neurotransmitter characteristics of neurons projecting to the supramammillary nucleus of the rat. Anat Embryol (Berl). 1999. 200:377–392.
3. Wyss JM, Swanson LW, Cowan WM. Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammillary region of the hypothalamus. Anat Embryol (Berl). 1979. 156:165–176.
4. Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992. 326:595–622.
5. Wyss JM, Swanson LW, Cowan WM. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979. 4:463–476.
6. Vertes RP, McKenna JT. Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse. 2000. 38:281–293.
7. Zamir N, Palkovits M, Brownstein M. Distribution of immunoreactive Met-enkephalin-Arg6-Gly7-Leu8 and Leu-enkephalin in discrete regions of the rat brain. Brain Res. 1985. 326:1–8.
8. Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ. Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol. 1990. 292:1–53.
9. Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinás RR. A dopaminergic projection to the rat mammillary nuclei demonstrated by retrograde transport of wheat germ agglutinin-horseradish peroxidase and tyrosine hydroxylase immunohistochemistry. J Comp Neurol. 1992. 321:300–311.
10. Hayakawa T, Zyo K. Fine structure of the supramammillary nucleus of the rat: analysis of the ultrastructural character of dopaminergic neurons. J Comp Neurol. 1994. 346:127–136.
11. Leranth C, Kiss J. A population of supramammillary area calretinin neurons terminating on medial septal area cholinergic and lateral septal area calbindin-containing cells are aspartate/glutamatergic. J Neurosci. 1996. 16:7699–7710.
12. Borhegyi Z, Maglóczky Z, Acsády L, Freund TF. The supramammillary nucleus innervates cholinergic and GABAergic neurons in the medial septum-diagonal band of Broca complex. Neuroscience. 1998. 82:1053–1065.
13. Mizumori SJ, McNaughton BL, Barnes CA. A comparison of supramammillary and medial septal influences on hippocampal field potentials and single-unit activity. J Neurophysiol. 1989. 61:15–31.
14. Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994. 14:7040–7052.
15. Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004. 24:5758–5765.
16. Shahidi S, Motamedi F, Naghdi N. Effect of reversible inactivation of the supramammillary nucleus on spatial learning and memory in rats. Brain Res. 2004. 1026:267–274.
17. Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982. 9:321–353.
18. Pan WX, McNaughton N. The supramammillary area: its organization, functions and relationship to the hippocampus. Prog Neurobiol. 2004. 74:127–166.
19. Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995. 26:403–412.
20. Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008. 33:659–669.
21. Wirtshafter D, Stratford TR, Shim I. Placement in a novel environment induces fos-like immunoreactivity in supramammillary cells projecting to the hippocampus and midbrain. Brain Res. 1998. 789:331–334.
22. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1986. 2nd ed. New York: Academic Press.
23. Oh JK, Kim YS, Park HJ, Lim EM, Pyun KH, Shim I. Antidepressant effects of Soyo-san on Immobilization stress in ovariectomized female rats. Biol Pharm Bull. 2007. 30:1422–1426.
24. Donahue DA, Dougherty EJ, Meserve LA. Influence of a combination of two tetrachlorobiphenyl congeners (PCB 47; PCB 77) on thyroid status, choline acetyltransferase (ChAT) activity, and short- and long-term memory in 30-day-old Sprague-Dawley rats. Toxicology. 2004. 203:99–107.
25. Wahba ZZ, Soliman KF. Effect of stress on choline acetyltransferase activity of the brain and the adrenal of the rat. Experientia. 1992. 48:265–268.
26. Jiang F, Khanna S. Microinjection of carbachol in the supramammillary region suppresses CA1 pyramidal cell synaptic excitability. Hippocampus. 2006. 16:891–905.
27. Lee HC, Chang DE, Yeom M, Kim GH, Choi KD, Shim I, Lee HJ, Hahm DH. Gene expression profiling in hypothalamus of immobilization-stressed mouse using cDNA microarray. Brain Res Mol Brain Res. 2005. 135:293–300.
28. Djordjevic J, Vuckovic T, Jasnic N, Cvijic G. Effect of various stressors on the blood ACTH and corticosterone concentration in normotensive Wistar and spontaneously hypertensive Wistar-Kyoto rats. Gen Comp Endocrinol. 2007. 153:217–220.
29. Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996. 368:88–99.
30. McEwen BS. Corticosteroids and hippocampal plasticity. Ann N Y Acad Sci. 1994. 746:134–142.
31. Kellendonk C, Gass P, Kretz O, Schütz G, Tronche F. Corticosteroid receptors in the brain: gene targeting studies. Brain Res Bull. 2002. 57:73–83.
32. Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Ann N Y Acad Sci. 1994. 746:392–393.
33. McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999. 22:105–122.
34. McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995. 5:205–216.
35. de Groote L, Linthorst AC. Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience. 2007. 148:794–805.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download