Abstract
Alcohol abuse and its medical and social consequences are a major health problem in many areas of the world. The present study was conducted to evaluate the protective effect of methanolic fruit extract of Randia dumetorum (L.) on alcohol-induced liver damage in rats. Rats were divided into five different groups (n=6), group I served as a control, group II received ethanol (3 ml/100 g/day p.o.), group III served as standard group and received silymarin (50 mg/kg p.o.), group IV and V served as extract treatment groups and received 50 & 100 mg/kg methanolic extract of R. dumetorum. All the treatment protocols followed 30 days and after rats were sacrificed blood and liver were used for biochemical and histological studies, respectively. The activities serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), direct bilirubin (DB), total bilirubin (TB) and lipid peroxidation were statistically increased in rats exposed to alcohol while total protein and glutathione decreased compared to control rats. Treatment with R. dumetorum significantly decreased the elevated levels of ALT, AST, TG, DB, TB and lipid peroxidation compared to the group exposed to alcohol only. R. dumetorum significantly resulted in increased levels of total protein and reduced glutathione compared to the group that received alcohol only. Histology of the liver section of the animals treated with R. dumetorum improved the hepatotoxicity caused by alcohol. Hence the study concluded that R. dumetorum has potential hepatoprotective activity.
Go to : 
Liver disease is a common condition in which major amounts of parenchyma cells are replaced by fibrous connective tissue. It is one of the serious health problems throughout the world. Liver is the typical detoxifying organ of the body and is profoundly affected by external chemicals [1]. Alcohol is a well-established hepatotoxicant that induces a diffuse type of liver injury closely resembling human viral hepatitis [2]. Oxidative stress plays a vital role in the patho-genesis of alcohol associated liver injury. The peroxidation of endogenous lipids has been shown to be a major factor in the cytotoxic action of alcohol [3,4].
Chronic alcohol consumption increases the capacity of cytochrome P450 2E1 (CYP2E1) to oxidize ethanol up to 10-fold which consequently increases the prooxidative burden. Reactive oxygen species (ROS) generated during ethanol oxidation via CYP2E1 contributes to ethanol induced liver injury [5]. Although the pathogenesis of alcohol-induced liver disease remains the subject of debate [6], one factor that has been suggested as playing a central role in many pathways of alcohol-induced damage, and which has been the focus of much research is the excessive generation of these free radicals, which can result in a state called oxidative stress [7]. Numerous studies have indicated that excessive ethanol intake induces the mass production of free radicals in the body, which are considered to be associated with alcoholic liver disease [8]. The most important characteristic of toxic free radicals either in vivo or in vitro is peroxidation of lipids resulting in tissue damage and death of affected cells [9]. Several reports have implicated free radical-induced lipid peroxidation in the pathogenesis of alcohol induced liver toxicity [10,11]. Antioxidants play an important role in the protection of cells and tissues against free radical-mediated tissue injury [12]. Although significant progress has been made in understanding the pathogenesis of alcoholic liver diseases, current therapies for these diseases are not effective. At present, except for abstinence from alcohol intake, there is no effective modality of either prevention or treatment [13,14]. Antioxidants of plant origin have been reported to either inhibit or prevent the development of fundamental cellular disturbances resulting from excessive alcohol consumption [15].
Randia dumetorum Lamk. (Rubiaceae) known as Madana (Sans), Mainphal (Hindi), Emetic nut (Eng) [16] is a small tree found in India in the tropical and subtropical region. Its fruits are considered to be tonic, alterative, demulcent, diuretic and restorative. The drug is claimed as a medical cure for piles, antidysentric agent, asthma, jaundice, diarrhea, emetic and gonorrhea [17].
Silymarin is a standardized seed extract of Silybum marianum, which contains flavonolignans. Silymarin at doses up to 100 mg/kg has been used as a standard hepatoprotective agent by numerous investigators. Dose of 50 mg/kg of silymarin was selected in the present investigation based on some published studies demonstrating the hepatoprotective activity of this dose [18]. In the present study, silymarin was used as the standard to compare the activity of the extract.
The literature survey revealed that there are no scientific studies carried out on hepatoprotective activity of the R. dumetorum. Hence the present study was designed to evaluate the chronic alcohol-induced liver oxidative damage and the efficacy of pretreatment of methanolic extract of Randia dumetorum (L.) fruit on chronic alcohol induced liver damage.
Go to : 
The fruits were collected from local area of Nashik, (Maharashtra) in month of April 2011. The fruit was authenticated by Dr. B.S. Baghel, Department of Botany, Krishi Vigyan Kendra Horti culture College, Mandsaur, Madhya Pradesh, India. The voucher specimen (MIP/P'cology/VSN-TS-23) was deposited at Department of Pharmacology, Mandsaur, Madhya Pradesh, India.
The fruits were air-dried in shade and powdered with a mechanical grinder to obtain a coarse powder. Maceration of air-dried powdered fruits of R. dumetorum afforded (17% w/w) methanol extract [19]. The methanolic extract was dried in vacuum evaporator below 40℃ and filtered through Whatmann filter no. 42 and reconstituted in saline.
The methanolic extract of R. dumetorum was subjected to phytochemical tests to identify the nature of chemical constituents present in the plant material [20].
Three months old male albino mice 20~40 g and male wistar rats 225~250 g were used to carry out acute toxicity studies and hepatoprotective activity respectively. The animals were procured from B.R. Nahata College of Pharmacy, Mandsaur. The animals were placed at random and allocated to treatment groups in polypropylene cages with paddy husk as bedding. Animals were housed at a temperature of 24±2℃ and relative humidity of 30~70%. A 12/12 h light and dark cycle (lights on at 8:00 a.m.) was followed. All animals were fed on standard balanced diet and provided with water ad libitum.
All the experimental procedures and protocols used in the study were reviewed and approved by the (IAEC) Institutional Animal Ethical Committee of Mandsaur Institute of Pharmacy, Mandsaur and were in accordance with the guidelines of the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA). Registration No.MIP/IAEC/2010/008
Acute toxicity studies were performed according to the OECD 425 guideline on albino mice and the animals were kept fasting for overnight providing water ad libitum. Animal shows toxic effect or mortality in an observation period of 48 hours at the doses of 1.75, 5.5, 17.5, 55, 175, 550 and 2,000 mg/kg. The number of deaths within this period was recorded. Effective dose (ED50) of extract was selected based on lethal dose (LD50) obtained from acute toxicity studies.
Thirty male albino wistar rats (200~230 g) were used. All the animals were divided into the five groups and they received the treatment for 30 days as follows.
Group I: Control (1 ml/kg saline p.o.)
Group II: 28.50% alcohol (3 ml/100 g/day p.o.) for 30 days
Group III: Standard (silymarine 50 mg/kg p.o.)+Alcohol for 30 days
Group IV: Methanolic extract of R. dumetorum (50 mg/kg p.o.)+Alcohol for 30 days
Group V: Methanolic extract of R. dumetorum (100 mg/kg p.o.)+Alcohol for 30 days
Alcohol (28.50%) solution in distilled water was administered in a dose of 3 ml/100 g/day p.o. for 30 days in three divided doses. In all the groups body weight was monitored weekly.
At the end of experimental period, rats were anaesthetized with ether. Blood samples were collected from retro orbital venous plexus in nonheparinized tubes, centrifuged at 3,000 rpm for 20 min, and blood sera were collected and stored at 4℃ prior immediate determination of ALT, AST, TG, DB, TB and total protein. All of these parameters were measured using Automated Clinical Chemistry Analysis System, Dimension type RXL Max (Dade Behring Delaware, DE 19714, USA).
The morphological parameters, liver weight and volume were determined. The liver weight was determined by using an electronic balance. The liver volume was determined by dropping the liver in a measuring cylinder containing a fixed volume of distilled water and the volume displaced was recorded.
The livers were removed and used for the assay of lipid peroxidation and reduced glutathione.
Lipid peroxidation was determined by thiobarbituric acid reactive substances (TBARS) method [23]. Briefly, liver samples were removed from sacrificed rats and placed in ice-cold 0.15 M KCL solution in a beaker embedded in salinated ice. The liver samples were rinsed thoroughly in the saline solution and excess fluid blotted out with a paper towel before weighing the samples in chilled containers. One gram portion of the liver was homogenized in 4 ml ice-cold 0.15 M KCL solution using a homogenizer. Liver homogenate (2 ml) was treated with 1 ml 2% TCA and then 2 ml 0.6% TBA in a ground glass tube. The lightly-stopper tube was warmed for 10 min in a boiling water bath. The mixture was centrifuged at 3,000 rpm for 10 min to remove precipitated proteins. The absorbance of a pink color produced by the reaction was read at 530 nM against water blank.
A known weight of tissue was homogenized in phosphate buffer. 0.5 ml of the homogenized mixture was treated with 1 ml 5% TCA, mixed and centrifuged. 2.0 ml of the supernatant was the treated with 1.0 ml Ellman's reagent and 4.0 ml 0.3 M disodium hydrogen phosphate. The absorbance of the yellow color developed was read in a UV spectronic spectrophotometer at 412 nM. A series of standards (20~100 µ/100 g) were treated in a similar manner along with a blank containing 1 ml buffer. The amount of glutathione was expressed as mg/100 g of protein.
Rats were sacrificed by cervical dislocation and liver was separated, washed in ringer's solution and soaked in filter paper. Immediately the liver was stored at -20℃ used for histopathological studies. The hepatoprotective activity was confirmed through histopathological studies on liver of rats. For light microscopic examination, liver tissues from each group were fixed with 10% buffered formalin, embedded with paraffin. After routine processing, paraffin sections of each tissue were cut into 4 µM thickness and stained with haematoxylin and eosin, and were observed with a light microscope Leica DMLB for histopathological changes.
All the data expressed as mean±S.E.M and analyzed statistically using ANOVA followed by Dunnett test and compare with respective control group. A value was of p<0.05 was considered significant.
Go to : 
Phytochemical screening was done for methanolic extract [19]. Methanolic extract gave positive tests for flavonoids, alkaloids, phenolics, steroids, terpenoids, saponins, fatty acid and carbohydrates.
There was no mortality amongst the graded dose groups of animals and they did not show any toxicity or behavioral changes at a dose level of 2,000 mg/kg. This finding suggests that extract was safe in or non-toxic to rats up to 2,000 mg/kg. Hence, the ED50 200 mg/kg was selected based on LD50 of plant.
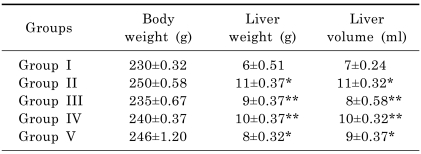
There were no significant differences about the body weights between the groups at any time. The animals slightly lost weight after seven days. They gained weight during the 30 days (Table 1).
The mean liver weight and volume was increased already after seven days postoperatively compared to controls and increased steadily further until day 30. The liver weight and volume were statistically (p<0.05) increased in rats exposed to alcohol compared with control group, and the histopathological lesions of the liver were evident. On administration of R. dumetorum or silymarin respectively, the level of these morphological parameters were found retrieving towards normalcy which is expressed in Table 1.
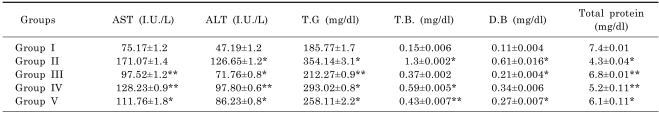
The result of effect of methanolic extract on enzyme and non-enzyme markers in alcohol treated rats is presented in Table 2. The activities in serum ALT, AST, TG, DB and TB were statistically (p<0.05) increased in rats exposed to alcohol while total protein decreased compared to control rats. Treatment with R. dumetorum or silymarin significantly (p<0.05) decreased the elevated levels of ALT, AST, TG, DB and TB compared to the group exposed to alcohol only. R. dumetorum or silymarin significantly (p<0.05) resulted in increased levels of total protein compared to the group that received alcohol only.
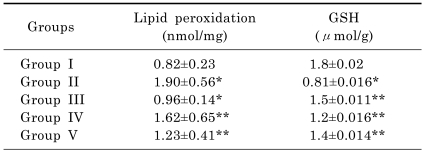
The results for the estimation of reduced glutathione and lipid peroxidation are presented in Table 3. The levels of reduced glutathione was significantly (p<0.05) lowered in group ingested with alcohol only. However, groups pretreated with 50 or 100 mg/kg of the methanolic extract or silymarin had significantly (p<0.05) elevated levels of reduced glutathione. Lipid peroxidation as assayed by TBARS was significantly (p<0.05) elevated in group ingested with alcohol only compared to control group. This effect was however, significantly (p<0.05) lowered by pretreatment with the methanolic extract or silymarin.
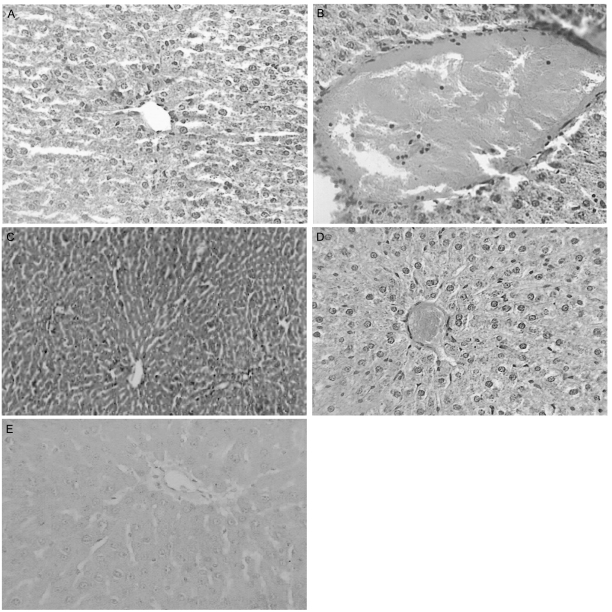
Light microscopic examination of the liver of control rats showed the normal structure in Fig. 1A. Histopathological effects of alcohol on liver of treated rats are presented in Fig. 1B. Rats treated with alcohol showing many severe histopathological alterations. Alcohol for 30 days resulted in the damage of liver structure along with disarrangement of hepatic strands. Several cells also show histological features of necrosis. Moreover, an enlargement of the sinusoids and vacuole formations in hepatocytes, leucocytic infiltrations, dilation, and congestion of blood vessels with hemorrhage were noted in liver of alcohol rats (group II). Silymarin-treated liver showing almost normal liver lobule with no sign of necrosis (Fig. 1C). Methanolic extract of R. dumetorum treatment brought back the cellular arrangement around the central vein and reduced necrosis (Fig. 1D). Also, it helped to bring the blood vessels to normal condition (Fig. 1E).
Go to : 
Alcohol is a well-established hepatotoxicant that induces a diffuse type of liver injury closely resembling human viral hepatitis. The prolonged intake ingestion of alcohol is associated with alcoholic hepatitis, fatty infiltration, and accelerated progression of liver disease and higher frequency of cirrhosis [25]. Escalating liver injury can lead to fibrosis and ultimately to cirrhosis.
In this view, the reduction in level of AST and ALT towards the normal value by the extract is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage caused by alcohol. This effect is in agreement with the commonly accepted view that serum level of transaminases returns to normal with the healing of hepatic parenchyma and regeneration of hepatocytes [26]. Serum bilirubin levels, on the other hand are related to hepatic cell damage. Effective control of bilirubin levels point toward an early improvement in the secretory mechanism of the hepatic cell. The protein level was also raised suggesting the stabilization of endoplasmic reticulum required for protein synthesis.
Alcohol administration in rats disrupts the membrane permeability of the plasma membrane causing leakage of the enzymes from the cells, which leads to the elevation of lipid peroxides. Lipid peroxidation is a free radical induced process leading to oxidative deterioration of polyunsaturated fatty acid. Under physiological condition, low concentrations of lipid-peroxides are found in tissues. It has been reported that the mechanisms of liver damage induced by alcohol is due to the instability of cellular membrane as a result of lipid peroxidation [27]. In the present study we have observed an increase in the levels of TBARS and hydroperoxides in the tissue of alcohol induced toxic rats. Lipid peroxidation arising from the reaction of free radicals with lipids is considered to be an important feature of the cellular injury brought by free radical attack [28]. Increased lipid peroxidation in various tissues has long been known to cause functional degradation. Thus, degradation of vital tissue leading to complications may be due indirectly to the increased oxidative stress. Administration of methanolic extract, in our study, significantly reduced the levels of TBARS. This observation demonstrates the antiperoxidative effect of methanolic extract on alcohol toxicity.
Cellular glutathione is a major component of the intracellular reducing machinery and a crucial factor of apoptosis [24]. Reduced glutathione acts as an antioxidant both intracellularly and extracellularly in conjunction with various enzymatic processes that reduce hydrogen peroxide and hydroperoxides by oxidizing reduced glutathione to its oxidized form and other mixed disulfides [29]. Ethanol administration has been reported to induce loss of glutathione from the liver and to cause a decrease in its hepatic content [30]. Glutathione, a tripeptide containing a sulfhydryl group, is a highly distinctive amino acid derivative with an important role in defending against lipid peroxidation. Since reduced glutathione inhibits lipid peroxidation in the liver, the decrease in GSH content in the liver could well give rise to an increase in malondialdehyde (MDA) formation, thus explaining the enhanced peroxidation in liver of ethanol-treated rats. Other factors that may contribute to a decrease in tissue-reduced glutathione include glutathione synthesis, utilization and limited intracellular reduction of oxidized glutathione to its reduced form [31]. Pretreatment of rats with methanolic extract of R. dumetorum prior to alcohol administration significantly prevented the depletion of the glutathione levels of rats compared to rats that ingested alcohol only. Increased levels of total glutathione could be the result of direct stimulation of antioxidant enzyme synthesis and increased levels of other antioxidants in the liver or enhanced reduction of oxidized glutathione. The availability of sufficient amount of reduced glutathione may enhance the detoxification of active metabolites through the involvement of glutathione peroxidase [32].
Chronic alcohol ingestion has been reported to result in increased lipid peroxidation through the formation of 4-hydroxy 2,3 neonenal, 4-hydroxy 2-3-alkanals and malondialdehyde [33]. Increased lipid peroxidation could be a direct consequence of decreased hepatic reduced glutathione content, which gives rise to an increase in malondialdehyde formation [11]. Escalation of lipid peroxidation in the liver due to chronic alcohol ingestion may be the result of increased formation of free radicals as well as the inhibition of antioxidant enzymes such as superoxide dismutase and catalase [34].
Decreased lipid peroxidation due to pretreatment with methanolic extract of R. dumetorum may be a reflection of the in vivo elevated levels of antioxidants. Increased level of reduced glutathione is known to protect against lipid peroxidation [12].
The histopathological observations in alcohol-treated rats showed severe necrosis, with disappearance of nuclei. This could be due to the formation of highly reactive radicals because of oxidative threat caused by alcohol. All these changes were very much reduced histopathologically in rats treated with methanolic extract of R. dumetorum.
The results of biochemical parameters (ALT, AST, TG, DB, TB and total protein) revealed the hepatoprotective activity of methanolic fruits extract of R. dumetorum against the toxic effect of alcohol, which was also supported by morphological (liver weight and volume) and histopathological studies. The phytochemical analysis of the extract has shown the presence of flavonoids, which has been known for their antioxidant and hepatoprotective activities [35]. Their activities are due to strong free radical scavenging property [36,37]. Therefore there is a possibility that the fruits of R. dumetorum may possess hepatoprotective activity. Histopathological changes in the liver sections also reveal the process of regeneration and reduction the necrosis.
In conclusion, the present study indicate that oral administration of methanolic fruit extract of R. dumetorum produces significant hepatoprotective effect in chronic alcohol treated rats. The overall hepatoprotective effect of R. dumetorum is probably due to a counteraction of free radicals by its antioxidants i.e. flavonoids. Further studies are needed to see if a higher dose and different routes of administration of R. dumetorum have a hepatoprotective effect.
Go to : 
ACKNOWLEDGEMENTS
Authors are thankful to Principal S. D. Parial, Mandsaur institute of pharmacy for providing the necessary facilities for carrying out this research work.
Go to : 
ABBREVIATIONS
ALT
alanine aminotransferase
AST
aspartate aminotransferase
TG
triglyceride
DB
direct bilirubin
TB
total bilirubin
TBARS
thiobarbituric acid reactive substances
GSH
reduced glutathione
ROS
reactive oxygen species
Go to : 
References
1. Song HS, Kim HR, Park TW, Cho BJ, Choi MY, Kim CJ, Sohn UD, Sim SS. Antioxidant effect of CoQ10 on N-nitrosodiethylamine-induced oxidative stress in mice. Korean J Physiol Pharmacol. 2009; 13:321–326. PMID: 19885017.
2. Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998; 59:533–543. PMID: 9718105.

3. Inoue M. Protective mechanisms against reactive oxygen species. 2001. 5th ed. Philadelphia: Lippincott Williams and Wilkins;p. 282–290.
4. Balasubramaniyan V, Manju V, Nalini N. Effect of leptin administration on plasma and tissue lipids in alcohol induced liver injury. Hum Exp Toxicol. 2003; 22:149–154. PMID: 12723896.
5. Younes M, Strubelt O. Alcohol-induced hepatotoxicity: a role for oxygen free radicals. Free Radic Res Commun. 1987; 3:19–26. PMID: 3508430.

6. Kessova I, Cederbaum AI. CYP2E1: biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr Mol Med. 2003; 3:509–518. PMID: 14527082.

7. Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002; 65:166–176. PMID: 11812920.

8. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993; 328:1828–1835. PMID: 8502273.
9. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003; 27:277–284. PMID: 15540798.
10. Ishii H, Kurose I, Kato S. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J Gastroenterol Hepatol. 1997; 12:S272–S282. PMID: 9407347.

11. Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: Oxidative damage and pathogenesis. Current Sci. 1999; 77:658–666.
12. Nadro MS, Arungbemi RM, Dahiru D. Evaluation of Moringa oleifera leaf extract on alcohol-induced hepatotoxicity. Trop J Pharm Res. 2006; 5:539–544.

13. Park JC, Hur JM, Park JG, Kim SC, Park JR, Choi SH, Choi JW. Effects of methanol extract of Cirsium japonicum var. ussuriense and its principle, hispidulin-7-O-neohesperidoside on hepatic alcohol-metabolizing enzymes and lipid peroxidation in ethanol-treated rats. Phytother Res. 2004; 18:19–24. PMID: 14750195.
14. Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002; 40:1213–1232. PMID: 13677623.
15. Zhou Z, Sun X, James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med (Maywood). 2002; 227:214–222. PMID: 11856821.

16. Gupta S, Pandey R, Katyal R, Aggarwal HK, Aggarwal RP, Aggarwal SK. Lipid peroxide levels and antioxidant status in alcoholic liver disease. Indian J Clin Biochem. 2005; 20:67–71.

17. Caliş I, Yürüker A, Taşdemir D, Wright AD, Sticher O, Luo YD, Pezzuto JM. Cycloartane triterpene glycosides from the roots of Astragalus melanophrurius. Planta Med. 1997; 63:183–186. PMID: 9140236.
18. Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997; 26:643–649. PMID: 9303494.

19. Kirtikar KR, Basu BD. Indian medicinal plants. 1999. 2nd ed. India: International Book Distributors Dehradun;p. 1760–1764.
20. Khandelwal KR. Practical pharmacognosy techniques and experiments. 2003. 10th ed. Pune: Nirali Prakashan;p. 149–158.
21. OECD. Guidelines for the testing of chemicals. 2001. Paris: OECD;p. 1–24.
22. Kumar S, Rao VN, Gouda TS. Hepatoprotective activity of alcoholic and aqueous extract of leaves of Tylophora indica (Linn) in rats. Indian J Pharmacol. 2004; 37:43–47.
23. Snell K, Mullock B. Biochemical toxicology: A practical approach. 1987. 2nd ed. Oxford: IRL press;p. 76–84.
24. Kaviarasan S, Ramamurty N, Gunasekaran P, Varalakshmi E, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006; 41:267–273. PMID: 16574673.

25. Wu YS, Salmela KS, Lieber CS. Microsomal acetaldehyde oxidation is negligible in the presence of ethanol. Alcohol Clin Exp Res. 1998; 22:1165–1169. PMID: 9726291.

26. Raja S, Ahamed KF, Kumar V, Mukherjee K, Bandyopadhyay A, Mukherjee PK. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J Ethnopharmacol. 2007; 109:41–47. PMID: 16930896.

27. Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997; 257:59–84. PMID: 9028626.

28. Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002; 27:63–68. PMID: 12062639.

29. Hung MY, Fu TY, Shih PH, Lee CP, Yen GC. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl4-induced hepatic damage in rats. Food Chem Toxicol. 2006; 44:1424–1431. PMID: 16707202.

30. Nadro MS, Arungbemi RM, Dahiru D. Evaluation of Moringa oleifera leaf extract on alcohol-induced hepatotoxicity. Trop J Pharm Res. 2006; 5:539–544.

31. Kabuto H, Hasuike S, Minagawa N, Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003; 93:31–35. PMID: 12865045.

32. Speisky H, MacDonald A, Giles G, Orrego H, Israel Y. Increased loss and decreased synthesis of hepatic glutathione after acute ethanol administration. Turnover studies. Biochem J. 1985; 225:565–572. PMID: 3977847.

33. Bhandarkar MR, Khan A. Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J Ethnopharmacol. 2004; 91:61–64. PMID: 15036469.

34. Jurczuk M, Brzóska MM, Moniuszko-Jakoniuk J, Gałazyn-Sidorczuk M, Kulikowska-Karpińska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol. 2004; 42:429–438. PMID: 14871584.

35. Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999; 65:337–353. PMID: 10421421.

36. Allan L, Miller ND. Antioxidant flavonoids: Structure, function and clinical usage. Alternative Med Rev. 1996; 1:103.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download