Abstract
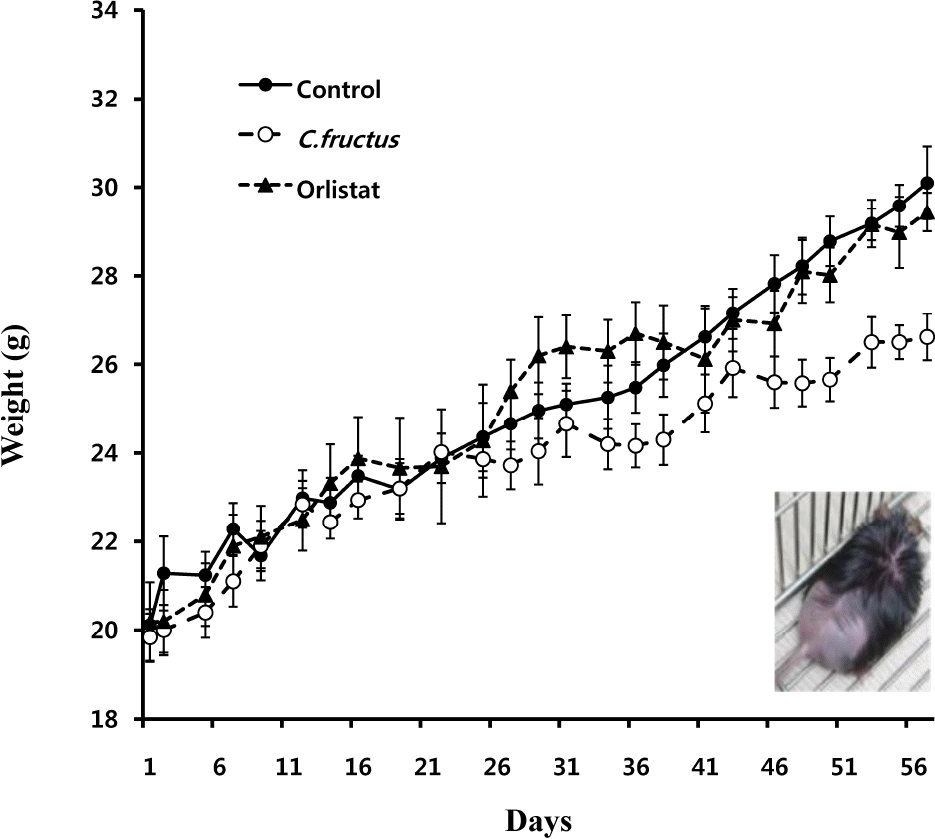
Natural products in Chonnam, Korea were screened via anti-angiogenesis experiments, and 1 candidate product was identified, Corni fructus, which exerted dose-dependent inhibitory effects against angiogenesis, adipogenesis, and cell adhesion. C. fructus extract (CFE) exhibits an angiogenesis inhibitory effect superior to that of the EGCG from green tea leaves. The expression level of angiogenesis and adipogenesis-related signal molecules in the western blotting was reduced by increasing the amount of added CFE. Moreover, a diet supplemented with CFE was deemed more effective in inducing weight loss in LB mice than a representative synthetic diet drug, orlistat, which incidently caused the side effect of denuding the mice of their hair. These results indicate that C. fructus may prove to be a useful anti-adipogenic compound, and these in vitro results may be reflected later under in vivo conditions.
Go to : 
References
1. Han DH, Kim SK, Kang SW, Choe BK, Kim KS, Chung JH. Matrix metallopeptidase 2 gene polymorphism is associated with obesity in Korean population. Korean J Physiol Pharmacol. 2008; 12:125–129.

2. Jung MY, Kim BS, Kim YJ, Koh IS, Chung JH. Assessment of relationship between fyn-related kinase gene polymorphisms and overweight/obesity in Korean population. Korean J Physiol Pharmacol. 2008; 12:83–87.

3. Pataky Z, Bobbioni-Harsch E, Golay A. Obesity: a complex growing challenge. Exp Clin Endocrinol Diabetes. 2010; 118:427–433.

4. Frezza EE, Wachtel MS, Ewing BT. The impact of morbid obesity on the state economy: an initial evaluation. Surg Obes Relat Dis. 2006; 2:504–508.

6. Doldi N, Bassan M, Gulisano M, Broccoli V, Boncinelli E, Ferrari A. Vascular endothelial growth factor messenger ribonucleic acid expression in human ovarian and endometrial cancer. Gynecol Endocrinol. 1996; 10:375–382.

8. Crandall DL, Hausman GJ, Kral JG. A review of the micro-circulation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997; 4:211–232.

9. Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004; 82:925–934.
10. Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004; 18:983–985.

11. Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005; 146:4545–4554.

12. Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010; 318:2–9.

13. Rudkowska I, Roynette CE, Demonty I, Vanstone CA, Jew S, Jones PJ. Diacylglycerol: efficacy and mechanism of action of an anti-obesity agent. Obes Res. 2005; 13:1864–1876.

14. Obeid OA, Bittar ST, Hwalla N, Emery PW. Effect of diet supplementation with glutamine, dihydroxyacetone, and leucine on food intake, weight gain, and postprandial glycogen metabolism of rats. Nutrition. 2005; 21:224–229.

15. Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007; 19:612–617.
16. Yun JW. Possible anti-obesity therapeutics from nature–a review. Phytochemistry. 2010; 71:1625–1641.
17. Oh S, Kim KS, Chung YS, Shong M, Park SB. Anti-obesity agents: a focused review on the structural classification of therapeutic entities. Curr Top Med Chem. 2009; 9:466–481.

18. Lee SO, Kim SY, Han SM, Kim HM, Ham SS, Kang IJ. Corni fructus scavenges hydroxy radicals and decreases oxidative stress in endothelial cells. J Med Food. 2006; 9:594–598.

19. Choi WH, Chu JP, Jiang MH, Baek SH, Park HD. Effects of fraction obtained from Korean Corni Fructus extracts causing anti-proliferation and p53-dependent apoptosis in A549 lung cancer cells. Nutr Cancer. 2011; 63:121–129.

20. Park CH, Noh JS, Tanaka T, Yokozawa T. Effects of morroniside isolated from Corni Fructus on renal lipids and inflammation in type 2 diabetic mice. J Pharm Pharmacol. 2010; 62:374–380.

21. Yamabe N, Noh JS, Park CH, Kang KS, Shibahara N, Tanaka T, Yokozawa T. Evaluation of loganin, iridoid glycoside from Corni Fructus, on hepatic and renal glucolipotoxicity and inflammation in type 2 diabetic db/db mice. Eur J Pharmacol. 2010; 648:179–187.

22. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992; 97:493–497.
23. Lee DH, MacIntyre JP, Wang E, Hudson DJ, Ishaque A, Conant JA, Pope BL, Lau CY. A leukocyte lipid up-regulates the avidity of lymphocyte function-associated antigen-1. Biochem Biophys Res Commun. 1994; 199:319–326.

24. Vandermeeren M, Janssens S, Borgers M, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun. 1997; 234:19–23.

25. Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999; 98:147–157.

26. Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem. 2004; 279:19683–19690.
27. Ballinger A, Peikin SR. Orlistat: its current status as an anti-obesity drug. Eur J Pharmacol. 2002; 440:109–117.

28. Jandacek RJ, Woods SC. Pharmaceutical approaches to the treatment of obesity. Drug Discov Today. 2004; 9:874–880.

29. Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 2007; 12:879–889.

30. Weigle DS. Pharmacological therapy of obesity: past, present, and future. J Clin Endocrinol Metab. 2003; 88:2462–2469.

31. Choi YS, Park H, Jeong S. Distinct role of PI3-kinase/Akt pathway in the activation of etoposide-induced NF- B transcription factor. J Microbiol Biotechnol. 2006; 16:391–398.
32. Tang FY, Nguyen N, Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int J Cancer. 2003; 106:871–878.
Go to : 
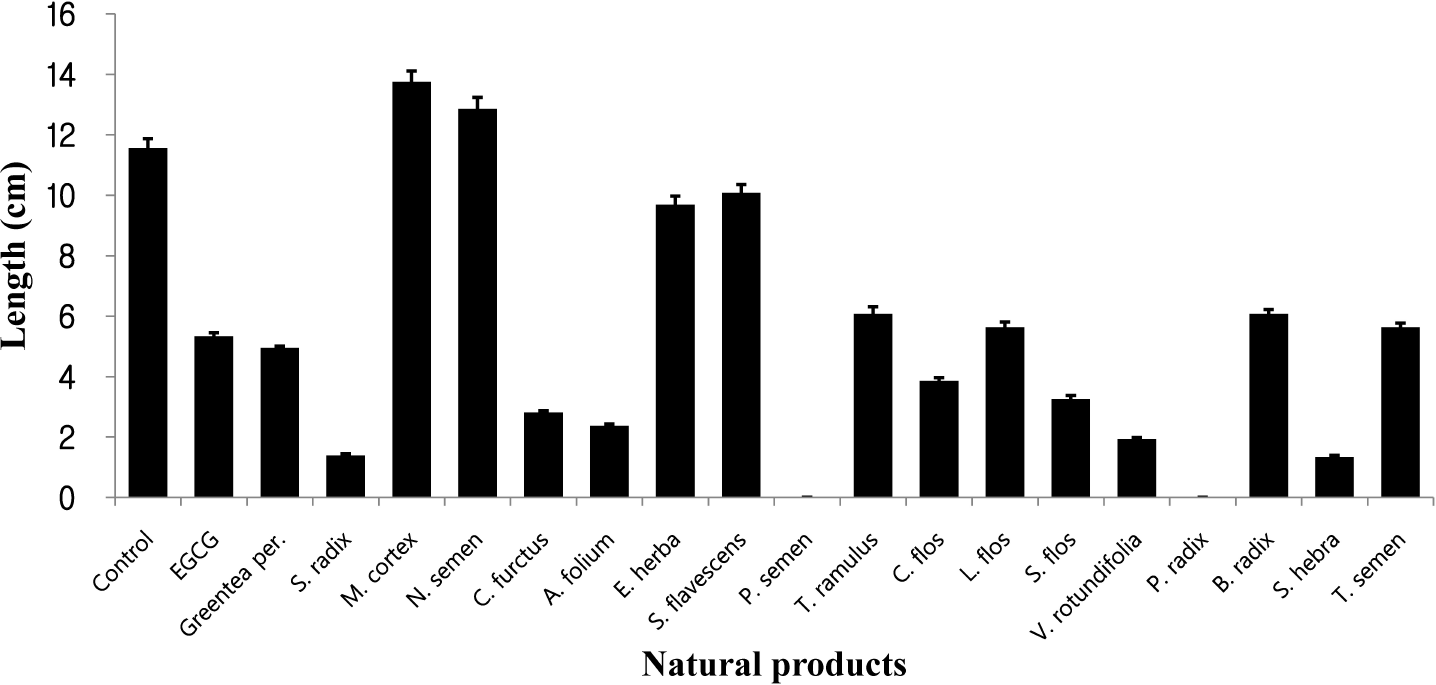 | Fig. 1.Anti-angiogenesis experiment of 18 natural products with the positive control, EGCG. HUVEC was cultured on Matrigel-coated wells, and the tube formation of HUVEC cells was randomly photographed using a digital camera, and analyzed by the Scion Image software. |
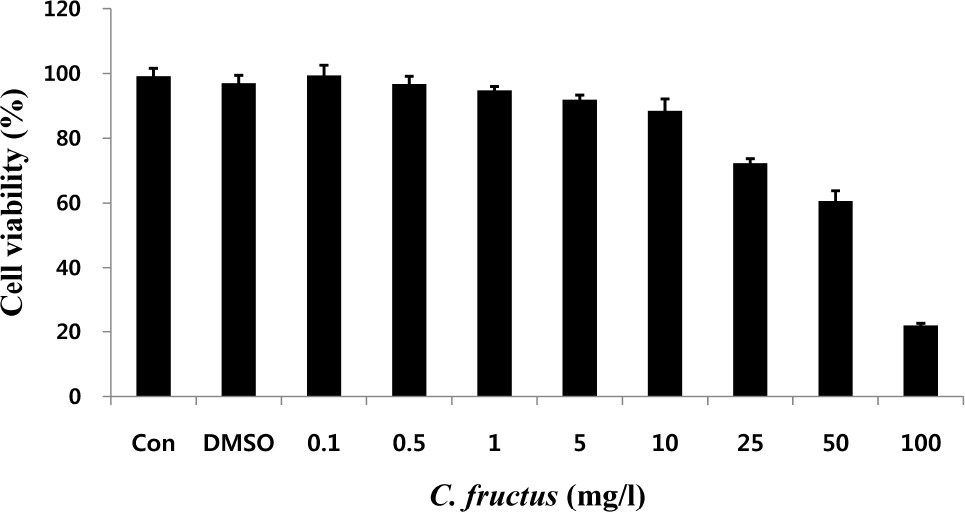 | Fig. 2.Cytotoxicity in HUVEC cells with different concentrations of C. fructus extract (CFE). HUVEC was seeded onto 96-well plates and treated with various doses (0.1, 0.5, 1, 5, 10, 25, 50, and 100 mg/l) of CFE for 48 hrs. MTT solution was added to the wells, and the 96-well plates were incubated. The absorbances of the 96-well plates were measured with a microplate reader at 540 nm. The value was converted to cell viability based on the control. |
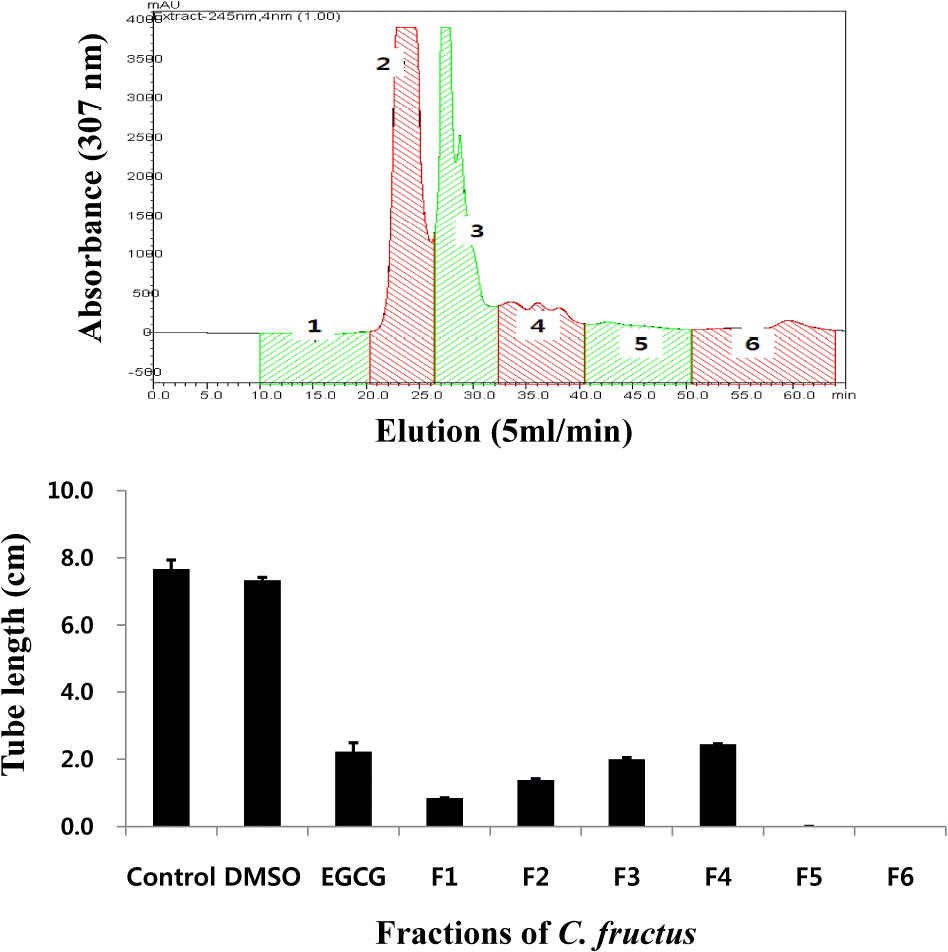 | Fig. 3.CFE was subjected to preparative size exclusion column of Asahipak GS-310. CFE was chromatographed on an Asahipak GS-310 column eluted with methanol at a flow rate of 5 ml/min, and monitored at 307 nm. CFE was separated into six fractions. Each fraction was evaluated via anti-angiogenesis experiments. HUVEC was cultured on Matrigel-coated wells, and the tube formation of HUVEC cells was randomly photographed using a digital camera, and analyzed by the Scion Image software. |
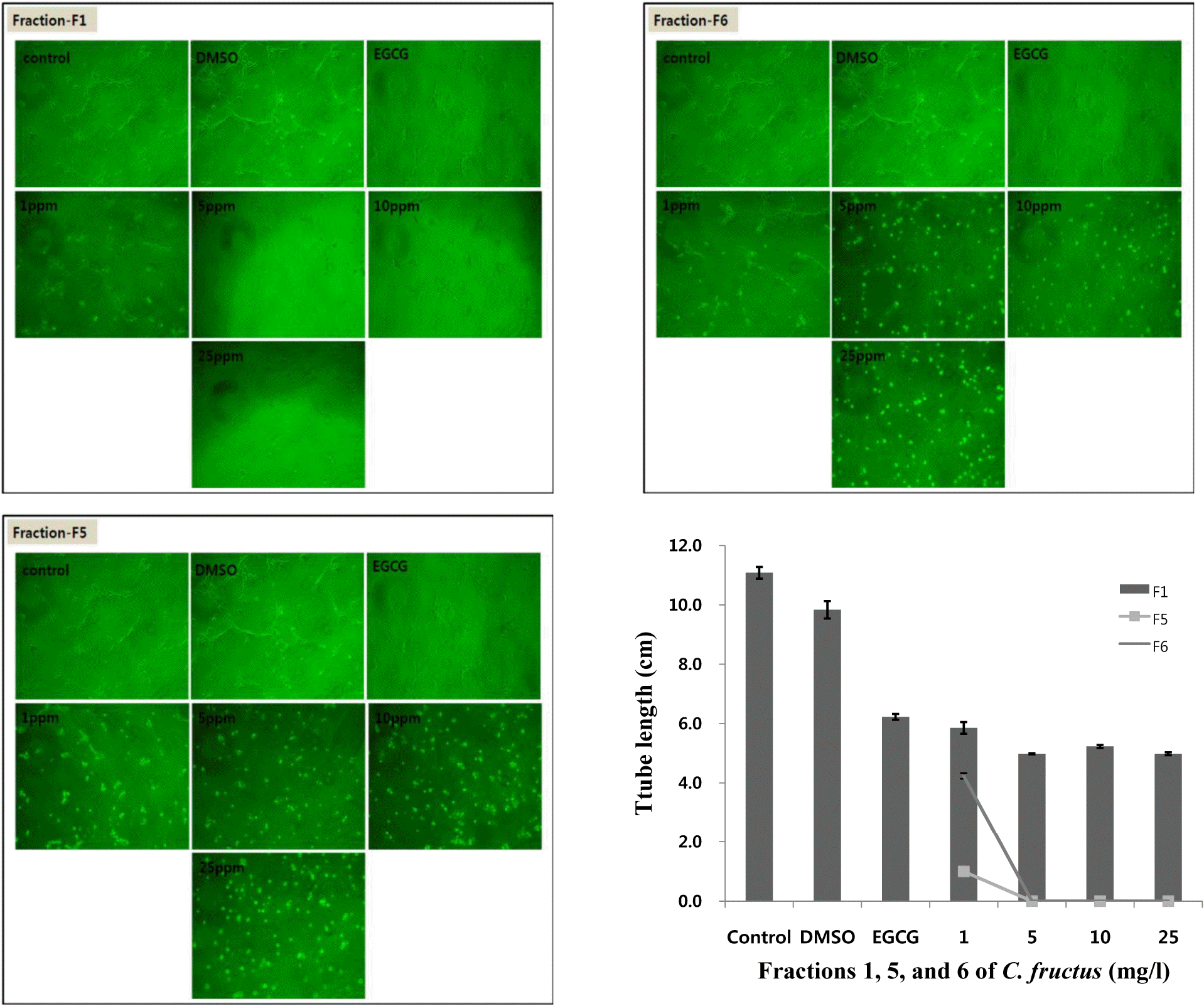 | Fig. 4.Anti-angiogenesis effect of SHE fractions 1, 5, and 6. Anti-angiogenesis experiments were conducted with different concentrations (1, 5, 10, and 25 ppm) of fractions 1, 5, and 6. The pictures show the cellular morphology of HUVEC cells treated with different concentrations of fractions 1, 5, and 6. |
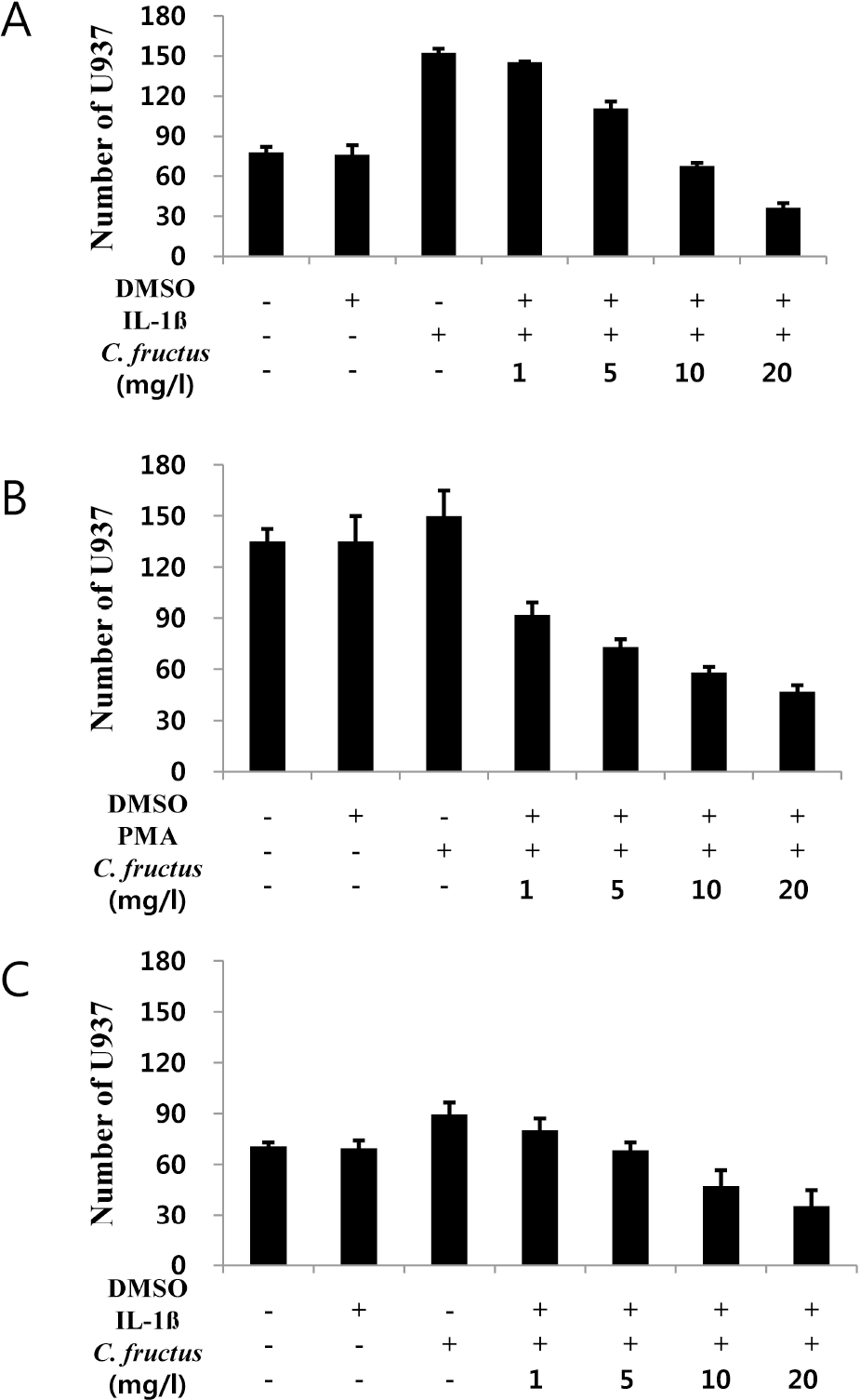 | Fig. 5.Various doses (1, 5, 10, and 20 mg/l) of CFE addition on U937 cell adhesion. (A) Cell adhesion of U937 on IL-1β and CFE stimulated HUVEC, (B) Cell adhesion of PMA- and CFE-stimulated U937 on HUVEC, (C) Cell adhesion of CFE-stimulated U937 on IL-1β- and CFE-stimulated HUVEC cells. Each treatment in the cell adhesion experiments was conducted as described in the METHODS section. |
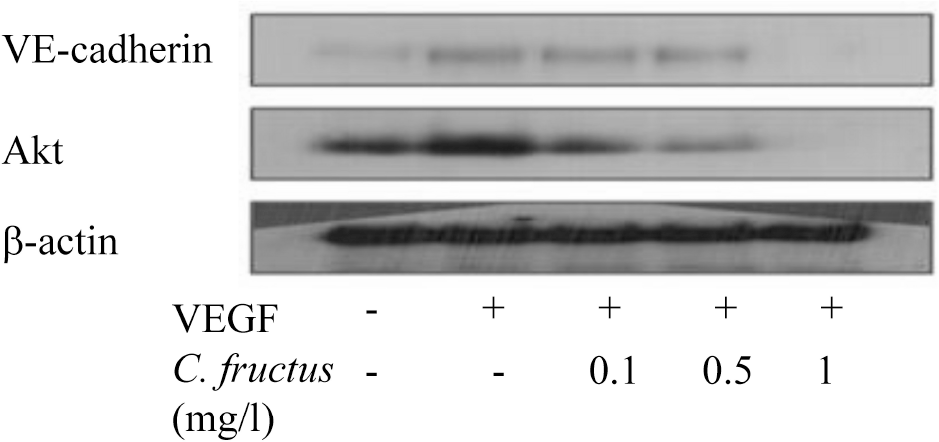 | Fig. 6.CFE inhibits the interaction of PI3-kinase with VE-cadherin and Akt upon cell activation with VEGF. Proteins from HUVEC cells were immunoprecipitated with PI3-kinase antibody and immunoblotted with antibodies to VE-cadherin and Akt. β-actin was employed as a positive control. |
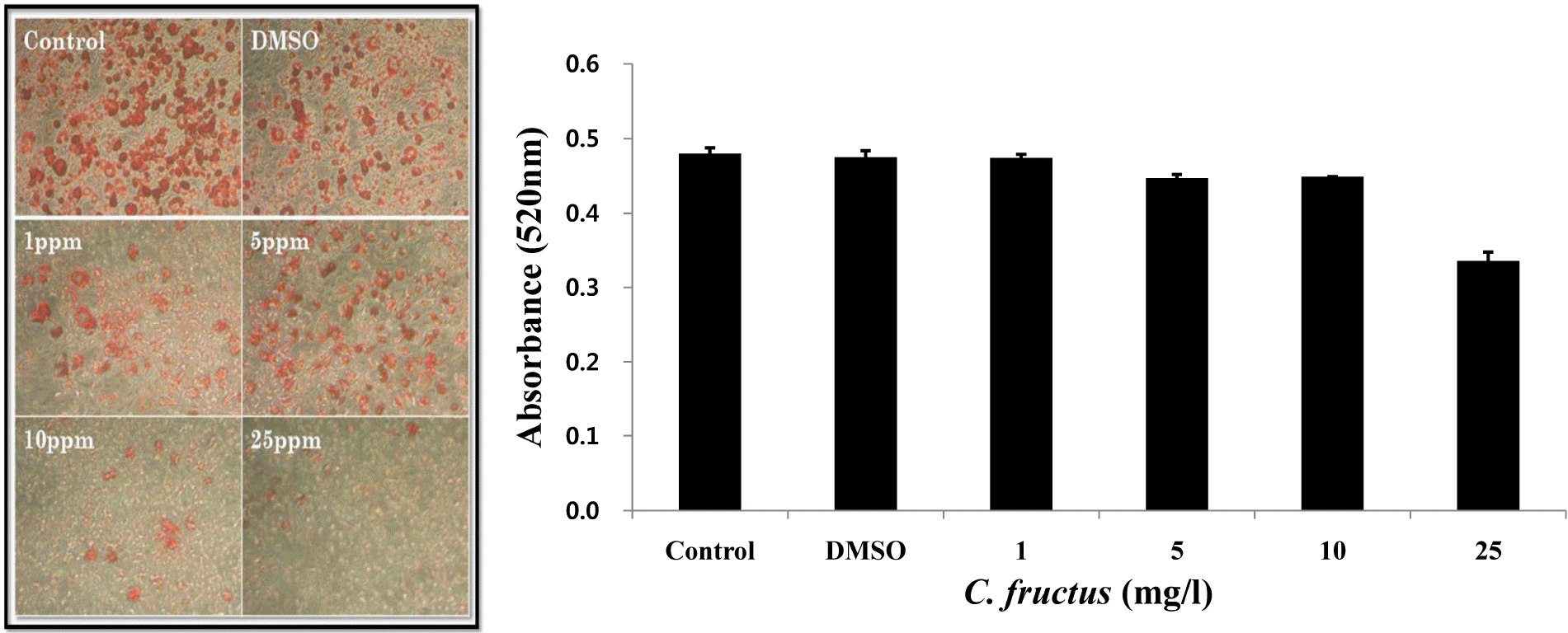 | Fig. 7.The effect of CFE addition in the course of 3T3-L1 adipocyte differentiation. 3T3-L1 was treated with CFE at various concentrations (1, 5, 10, and 25 mg/l). After differentiation, lipid accumulation was stained with Oil red O solution and the morphological changes were observed via microscopy. The Oil red O stained lipid was quantified with a microplate reader at 520 nm. |
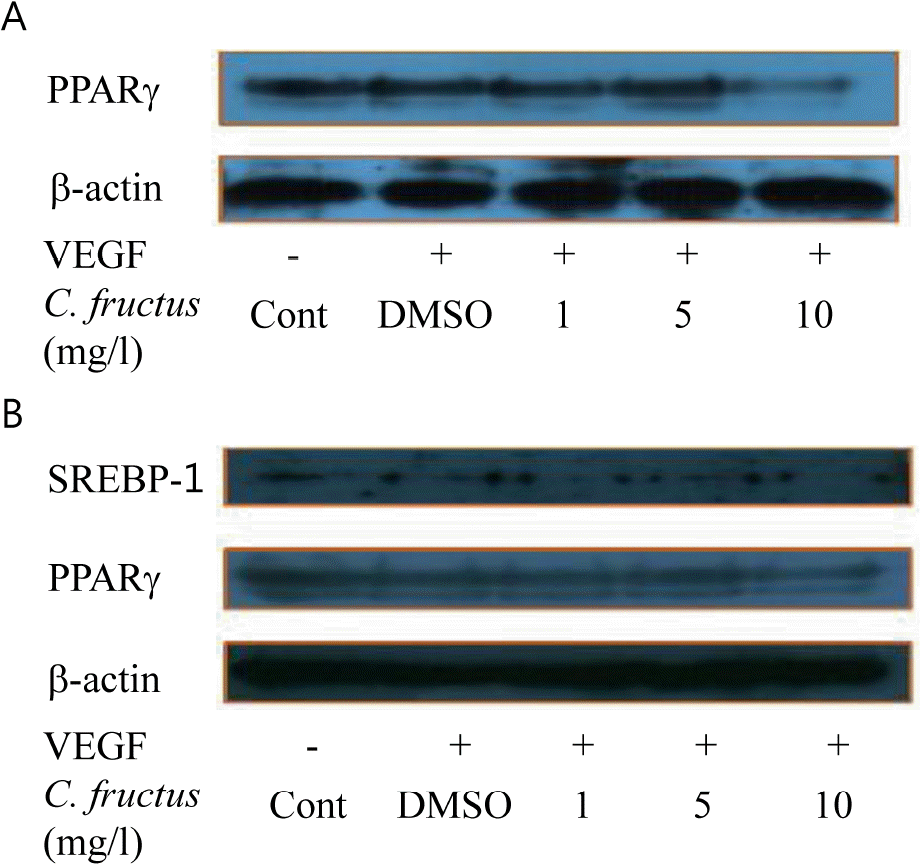 | Fig. 8.(A) CFE inhibits the interaction of PI3-kinase with VE-cadherin and Akt upon cell activation with VEGF. Proteins from HUVEC cells were immunoprecipitated with PI3-kinase antibody and immunoblotted with antibodies to VE-cadherin and Akt. β-actin was employed as a positive control. Expression of PPARγ on 3T3-L1 preadipocyte with CFE (A) and expression of SREBP-1 and PPARγ on 3T3-L1 adipocytes with CFE (B). Protein from 3T3-L1 was treated with different concentrations (1, 5, and 10 mg/l) of CFE, and extracted with RIPA buffer for western blotting. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download