INTRODUCTION
METHODS
In vitro recording of retinal activity
Electrode and data recording system
Data analysis
RESULTS
Spontaneous hyperactivity in degenerated retina; rd1 and rd10 retina
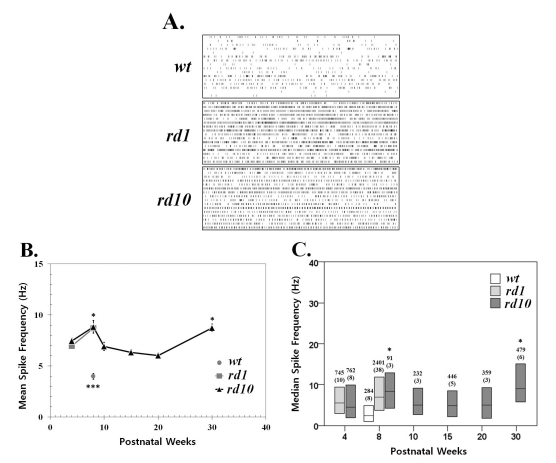 | Fig. 1Spontaneous activity increases with photoreceptor degeneration in rd1 and rd10 ganglion cells. (A) Representative raster plots display extracellular recordings of spontaneous action potentials in wild type (wt), rd1, and rd10 at postnatal ages of 8 weeks. Each panel displays the RGC activity recorded over 10 sec period in a sample of 16 ganglion cells (1 per row) from a single retina. (B) Mean±SEM firing rate of all cells recorded across different postnatal ages. Spontaneous activity significantly increases with aging from postnatal 4 weeks to 8 weeks both in rd1 and rd10 ganglion cells (p<0.05). At PNW8, the mean spike frequency of wt is significantly lower than either rd1 or rd10 (***p<0.001). In rd10, the mean firing rate at PNW8 and PNW30 are highest, and no statistical difference among the other age groups (ANOVA, p=0.05, posthoc Tukey criteria). Statistically significant differences among different age groups are indicated above the mean value (*p<0.05). (C) Median spontaneous firing rates (middle line in each bar) and 1st and 3rd quartiles (ends of bars) are indicated for each of 6 age groups. The median spike rate at PNW8 is the highest, and at PNW30, second highest, and no difference among PNW4, 10, 15, and 20 (Kruskal-Wallis test, p=0.05, Tukey's criteria). Statistically significant differences among different age groups are indicated above the median value (*p<0.05). Data are from the same recordings in B. The number of cells in each sample is indicated above the bar, with the number of retinas in parentheses. Each retina was from a different animal. |
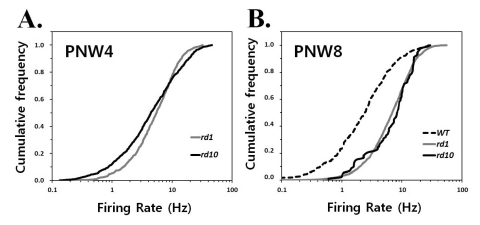 | Fig. 2Quantification of spontaneous hyperactivity in rd1 and rd10 ganglion cells. Cumulative frequency histograms display the distribution
of mean firing rates among all ganglion cells recorded in normal and degenerating retinas at postnatal 4 weeks (PNW4) (A) and 8 weeks (PNW8) (B) The same cells were used as in Fig. 1. Each panel represents the proportion of the ganglion cell population firing at a rate up to the frequency of x axis. A greater proportion of cells is active at higher rates in rd1 and rd10 mice comparing with wt mice, seen as a rightward shift and steeper slope of the histogram curve. |
Rhythmic local field potential and bursting RGC spikes in rd1 and rd10 retina
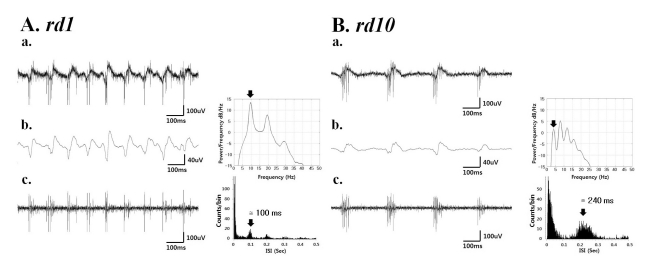 | Fig. 3(a) A typical raw waveform of neural activity recorded from rd1 (A) and rd10 (B) retina. (b) Field potential waveform (left) obtained from low-pass filtering with 20 Hz cutoff frequency and its power spectral density estimated by the Burg algorithm [23] (right). (c) The spiking activity obtained by high-pass filtering with 100 Hz cutoff frequency (left) shows a temporal structure of rhythmic bursts of spikes, where the interburst interval of ~100 ms (corresponding to ~10 Hz) and ~240 ms (corresponding to ~4 Hz) in rd1 and rd10 retina, respectively. |
Rhythmic local field potential and bursting RGC spikes across different age groups
1. Rhythmic burst of spontaneous RGC spikes is always phase-locked with the oscillatory field potential in rd1 retina (Fig. 4)
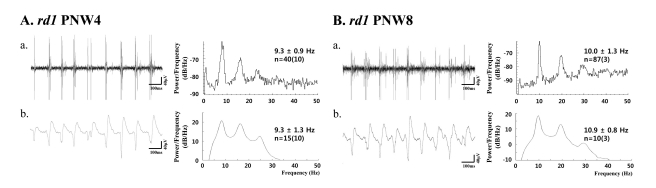 | Fig. 4(a) Bursting RGC spikes (left) and its power spectrum (right) (b) LFP (left) and its power spectrum (right) in rd1 retina at postnatal 4 weeks (PNW4) (A) and PNW8 (B). RGC spikes and LFP were obtained with high-pass filtering and low-pass filtering as in Fig. 3. The number of cells is indicated, with the number of retinas in parentheses. There is no statistical difference between the spectral peak in bursting RGC spikes and LFP (p>0.05) in both PNWs. |
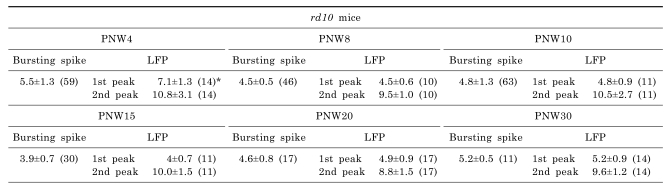
2. Rhythmic burst of spontaneous RGC spikes is phase-locked with the first spectral peak of the oscillatory field potential in rd10 retina (Fig. 5)
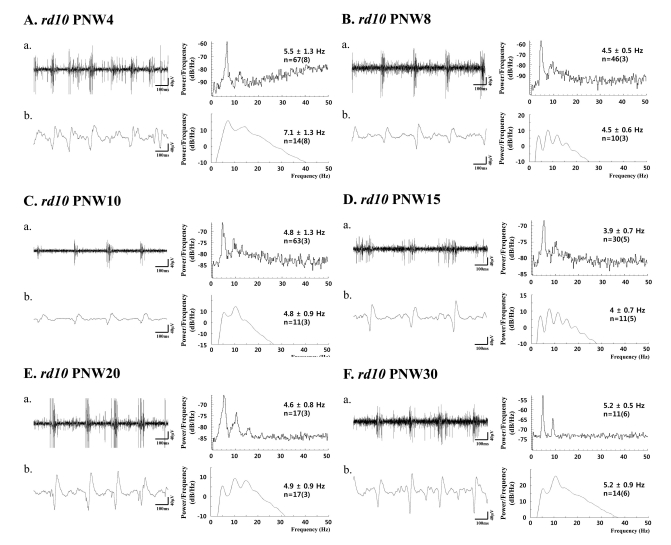 | Fig. 5(a) Bursting RGC spikes (left) and its power spectrum (right) (b) LFP (left) and its power spectrum (right) in rd10 retina with PNW4 (A), PNW8 (B), PNW10 (C), PNW15 (D), PNW20 (E), and PNW30 (F). RGC spikes and LFP were obtained with high-pass filtering and low-pass filtering as in Fig. 3 and 4. The number of cells is indicated, with the number of retinas in parentheses. Except PNW4, no statistical difference between the spectral peak of bursting RGC spikes and first peak of oscillatory field potential was found among different age groups (ANOVA, p>0.05). |
3. ~ 5 Hz frequency is found in continuous wavelet transform calculated from retinal activity for short recording time in rd10 retina
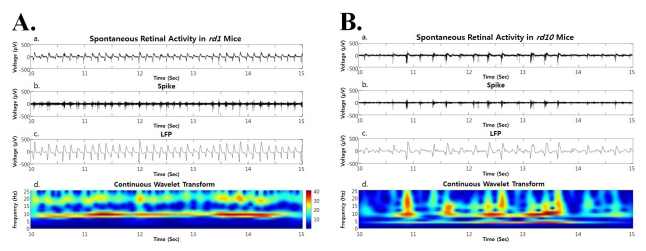 | Fig. 6Both in rd1 and rd10 mice, the spontaneous retinal activity (a), spike obtained by high pass filtering (b), LFP obtained by low pass filtering (c), and power spectrum through continuous wavelet transform are shown. (A) In rd1 mice, hot spot is only observed at ~10 Hz frequency (Ad). (B) In rd10 mice, hot spot is more prominent at ~10 Hz frequency but substantial hot spot is also found at ~5 Hz (Bd). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download