INTRODUCTION
Following the stimulation of G-protein coupled receptors (GPCRs) or receptor tyrosine kinases (RTKs) by hormones, neurotransmitters, stretch, or cytokines [
1] activated G
α subunits stimulate phospholipase C β (PLCβ), and phosphorylated RTKs stimulate PLCγ, respectively. Inositol 1,4,5-triphosphate (IP
3) generated by PLC [
2-
4] evokes Ca
2+ release from the endoplasmic reticulum (ER), and depletion of Ca
2+ from the ER activates the entry of store-operated Ca
2+[
1]. In this way, the cytosolic concentration of intracellular Ca
2+ is increased, which plays various roles in fertilization, proliferation, development, learning and memory, contraction and secretion, as well as initiation of apoptosis accompanied by cytotoxic effects [
1,
4,
5]. Finally, increased levels of Ca
2+ lead to activation of the sarco/ER Ca
2+ ATPase (SERCA), and the plasma membrane Ca
2+ ATPase (PMCA) pump removes Ca
2+ from the cytosol [
1,
4].
Ca
2+ oscillation is an important phenomenon in Ca
2+ signaling because a weak physiological stimulus generates transient repetitive oscillations in intracellular Ca
2+ concentration ([Ca
2+]
i) [
6]. There are two theories to explain Ca
2+ oscillation. One theory is based on an IP
3 receptor-related adaptation model. In this model, IP
3 affinity to IP
3 receptors is related to [Ca
2+]
i. Therefore, the generation of Ca
2+ oscillations is explained biophysically [
7]. The other theory is a biochemical model, which is explained by oscillations of intracellular amounts of IP
3; this phenomenon itself leads to Ca
2+ oscillations [
8]. In addition, regulators of G-protein signaling (RGS) proteins are regarded as components of this biochemical mechanism [
9].
Several types of RGS proteins exist; the homolog of RGS4 protein, SST2, was discovered first in
Saccharomyces cerevisiae [
10,
11]. Homologs of SST2 were found in various species, including
Caenorhabditis elegans [
12] and
Homo sapiens [
13]. RGS proteins were named because of their biochemical function of activating GTPases [
14]. RGS proteins inhibit GPCRs via their GTPase activity which accelerates GTP hydrolysis [
14]. In mammals, there are many members of the RGS protein family, and some types of RGS proteins have tissue specificity [
9].
Pancreatic acinar cells are a good model in which to study Ca
2+ signaling because cholecystokinin (CCK) and acetylcholine induce Ca
2+ oscillations that are initiated in the pancreatic secretory granules; these cells have characteristics that are regulated in both a spatial and temporal manner [
15,
16]. Exocytosis of secretory granules containing digestive enzymes occurs via Ca
2+ oscillations [
15-
17]. It is currently thought that RGS1, RGS2, RGS4, and RGS16 are expressed in pancreatic acinar cells [
18]. Increased steady-state levels of IP
3 were shown to lead to an increased frequency of [Ca
2+]
i oscillations in RGS2 knock-out mice [
19]. Nevertheless, the roles of other RGS proteins in pancreatic acinar cells have not been investigated. The GTPase accelerating activity of RGS4 can be stimulated by Homer 2, which preferentially binds PLCβ in pancreatic acinar extracts [
20]. In pancreatic islets, RGS4 deficiency had an effect on insulin release caused by the activation of other β-cell GPCRs. Additionally, treatment of mutant mice selectively lacking RGS4 in pancreatic β-cells treated with a muscarinic agonist (i.e., bethanechol) led to increased plasma insulin and reduced blood glucose levels [
21]. From these results, we inferred that RGS4 protein might have an important role in pancreatic acinar cells.
To reveal the function of RGS4 protein in pancreatic acinar cells, we investigated the mechanism of GPCR-induced Ca2+ signaling in pancreatic acinar cells derived from RGS4-/- mice. We found that mutant mice demonstrated more frequent Ca2+ signaling oscillations, more robust Ca2+ mobilization, and increased SERCA2b expression. Our findings suggest that RGS4 protein can regulate Ca2+ signaling in pancreatic acinar cells.
Go to :

METHODS
Materials and antibodies
Fura-2/AM was purchased from Teflabs (Austin, TX, USA); CCK octapeptides (sulfated) were purchased from Tocris Biosciences (Bristol, BS11 0QL, UK). Collagenase P was purchased from Roche (Indianapolis, IN, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Anti-PMCA (5F10) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-IP3R2 and anti-SERCA2b antibodies were from AbFrontier (Seoul, Korea). Anti-IP3R3 antibodies were from BD Transduction Laboratories (San Jose, CA, USA). Anti-β-actin was from Sigma-Aldrich.
Animals and preparation of pancreatic acinar cells
Wild-type (WT) and RGS4 mutant (RGS4
-/-) mice with a C57BL/6 background were purchased from Jackson Laboratories, All experiments were performed on adult male C57BL/6 or RGS4 knock-out mice (2 to 6 months of age) that were maintained on a 12-h day/night cycle with normal mouse chow and water provided
ad libitum. All procedures involving animals were performed according to the guidelines of the Yonsei University College of Dentistry, Intramural Animal Use and Care Committee. Mice were sacrificed by cervical dislocation under CO
2 anesthesia. The cells were prepared from the pancreases of WT and RGS4
-/- mice by limited collagenase digestion as previously described [
22]. Following isolation, the acinar cells were suspended in an extracellular physiologic salt solution (PSS), the composition of which was as follows (in mM): 140 NaCl, 5 KCl, 1 MgCl
2, 1 CaCl
2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with 10 N NaOH and to 310 mOsm with 5 M NaCl.
Measurement of [Ca2+]i
Pancreatic acinar cells from WT and RGS4-/- mice were loaded with 4μM fura-2/AM and 0.05% pluronic acid F-127 for 30 min in PSS at room temperature. Fura-2/AM fluorescence was measured at an excitation wavelength of 340/380 nm, and emission was measured at 510 nm (ratio=F340/F380) using an imaging system (Molecular Devices, CA, USA). The emitted fluorescence was monitored using a CCD camera (CoolSNAP HQ, AZ, USA) attached to an inverted microscope. Fluorescence images were obtained at 2-s intervals. All data were analyzed using the MetaFluor software (Molecular Devices, Downingtown, PA, USA).
Western blot
Protein extracts were prepared from isolated pancreatic acini from WT and RGS4-/- mice as follows. Pure acinar cells were lysed in a buffer containing (in mM): 150 NaCl, 10 Tris (pH 7.8 with HCl), 1 EDTA, 1% NP-40, 0.1% SDS, and a protease inhibitor mixture (2 Na3VO4, 10 NaF, 10μg/ml leupeptin, and 10μg/ml phenylmethylsulfonyl fluoride). The samples were probed overnight with 1:1,000 dilutions of antibodies against PMCA (5F10), IP3R2, IP3R3, SERCA2b, and β-actin at 4℃ and then separated by SDS-PAGE.
Amylase secretion assay
Animals were allowed water but starved for 24 h prior to the experiment. Each acinar cell was stimulated with equal concentrations of carbachol used in the [Ca
2+]
i measurement study. Acinar cells were incubated with carbachol for 20 min in a shaking incubator at 37℃ and 60 rpm. Acinar cells were lysed by sonication. The lysates were clarified by centrifugation at 13,000 rpm for 10 min. The total amylase content or content of amylase released into the medium was determined by the method described by Bernfeld [
23]. Aliquots of the incubation medium and the supernatants of the homogenized cells were incubated with a 0.5% starch suspension for 10 min at 37℃. Absorbance was measured at 540 nm. Amylase activity in the medium was expressed as a percentage of the total activity.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from isolated pancreatic acinar cells using Trizol reagent (Sigma-Aldrich) according to the manufacturer's instructions. RT-PCR was performed using a SuperScript III RT kit (Sigma-Aldrich) and oligo-dTs (Fermentas, MA, USA). For PCR analysis of SERCA2b, the following primers were used: forward: 5'-TCGGAATACAGCGAGGAGAACATT-3'; reverse: 5'-TCCCTGCCTCTGTGTGAGAATTAG-3'. PCR was initiated by a 5-min incubation of the samples at 94℃, preceded by 25 cycles of 30 s at 94℃, 30 s at 58℃ for annealing, and 30 s at 72℃. After 25 cycles, samples were incubated for 10 min at 72℃ for complete extension. No reverse transcription control was performed using the same protocol, except for a control with no reverse transcriptase added.
Data analysis and statistics
Results are expressed as the mean±S.E.M. The statistical significance of differences between groups was determined using the Student's t test. In statistical tests, p values less than 0.05 were considered significant.
Go to :

DISCUSSION
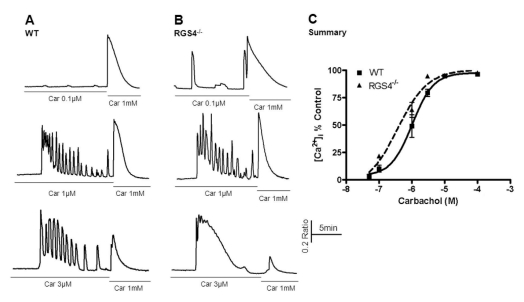
In this study, we demonstrated different Ca2+ signaling patterns in WT and RGS4-/- pancreatic acinar cells. First, Ca2+ mobilization was more prominent in pancreatic acinar cells of RGS4-/- mice compared with WT. This effect could be the result of reduced GTPase accelerating activity in RGS4-/- cells, which is the normal physiological function of the RGS4 protein. The effect could also be related to increased SERCA2b expression levels in RGS4-/- cells. Both factors could influence Ca2+ mobilization; however, based on our current experiments, we cannot conclude which is the major factor.
Nevertheless, this study indicates that RGS4
-/- pancreatic acinar cells are more sensitive to carbachol-induced Ca
2+ signaling. Therefore, we hypothesized that the frequency of Ca
2+ oscillations would increase in these cells. Results of the calcium mobilization test can be explained in the same manner. Ca
2+ oscillation is regarded as a key component in signal transmission and is related to the secretory process in pancreatic acinar cells [
15,
16]. Therefore, there would be an increase in the secretory function of cells that show an increase in the frequency of Ca
2+ oscillations.
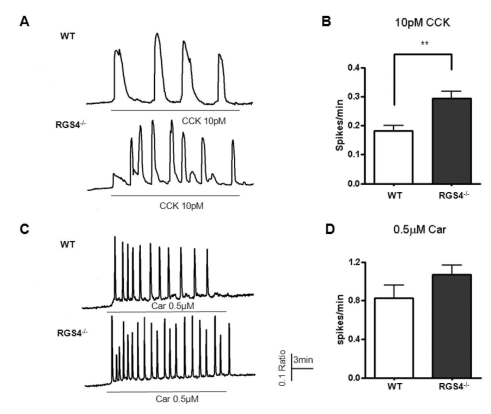
To confirm this hypothesis, we performed amylase secretion assays because pancreatic acinar cells secrete α-amylase during cytosolic Ca2+ oscillations. Unfortunately, we did not find any differences in amylase secretion between WT and RGS4-/- cells. This result indicates that the increase in Ca2+ oscillation frequency is insufficient to change the secretory function of the acinar cells.
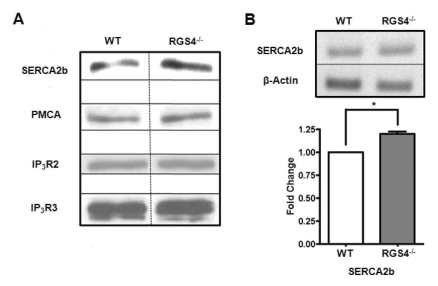
The next goal of this study was to evaluate the expression levels of molecules such as IP3R2, IP3R3, PMCA, and SERCA2b in WT and RGS4-/- acinar cells. A change in the frequency of Ca2+ oscillations was observed due to altered expression levels of these molecules. According to western blotting analysis, IP3R2, IP3R3, and PMCA exprxession levels were similar, but SERCA2b expression was slightly increased in RGS4-/- acinar cells. Quantitative PCR further confirmed these results, and these results suggest that deletion of RGS4 is probably related to SERCA2b gene expression in pancreatic acini cell. From these results, we inferred that deletion of RGS4 affects SERCA2b expression through an uncertain mechanism. We speculate that this phenomenon is an example of adaptation because cytosolic Ca2+ levels in RGS4-/- cells are higher and last longer than those in WT cells. In this situation, RGS4-/- cells need to clear Ca2+ ions from the cytosol more quickly.
The present study adds new insight into the signal transduction pathway of pancreatic acinar cells that regulate secretory function. RGS4 is a member of the B/R4 subfamily of RGS proteins, acting as a GTPase activating protein (GAP) for G
q- and G
i-type G-protein α-subunits [
24,
25]. In WT pancreatic acini, RGS4 inhibited certain G
q subunits, such as those in muscarinic receptors. For this reason, RGS4
-/- pancreatic acini may display enhanced activity of muscarinic receptors. Thus, increased Ca
2+ oscillation frequency and mobilization in RGS4
-/- acinar cells could be a result of function of RGS4.
And results of the increased SERCA2b expression, released Ca2+ would be quickly uptake to ER, and thereby makes the RGS4-/- acinar cells more ready for next stimulation. This change would be another explanation of increased frequency of Ca2+ oscillation.
In RGS4
-/- mouse pancreatic acinar cells, the frequency of Ca
2+ oscillations increased along with the induction of SERCA2b expression. In addition, levels of secretory amylase were slightly higher in RGS4
-/- cells. Elevated cytosolic Ca
2+ levels can induce cellular apoptosis [
5], and increased concentrations of cytosolic Ca
2+ lead to increased concentrations of Ca
2+ in the mitochondrial matrix, thereby inducing apoptosis [
26]. For these reasons, viable cells must clear cytosolic Ca
2+ rapidly. In RGS4
-/- pancreatic acini, more robust Ca
2+ release could be induced by physiologic stimuli when compared with WT cells. Hence, we suggest that the potential role of RGS4 in acinar cells is to regulate SERCA2b expression levels and the frequency of Ca
2+ oscillations.
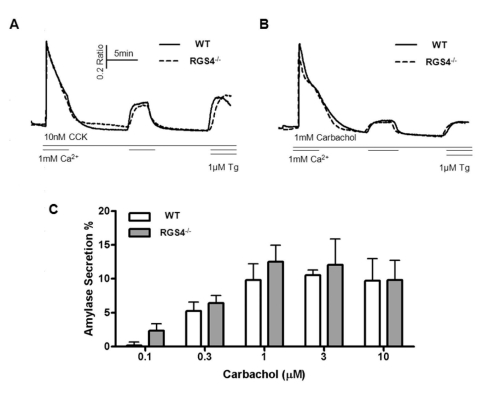
Increased activity of store-operated Ca
2+ channels (SOCs) induces Ca
2+ oscillations [
27]. In this context, increased frequency of Ca
2+ oscillations could be unrelated with SERCA2b expression. Nevertheless, in the present study, patterns of Ca
2+ influx/efflux were similar between WT and RGS4
-/- acinar cells after treatment with GPCR agonists. This result indicates that SOCs are not influenced by Ca
2+ signaling and that increases in the frequency of Ca
2+ oscillations would be due to increased expression of SERCA2b.
All alterations induced by RGS4 deletion in pancreatic acini could be a result of the modified physiologic function of pancreatic acinar cells. To confirm this hypothesis, we performed an amylase secretion assay. The average secretory value was slightly higher in RGS4-/- acinar cells than in WT cells, although the difference was not significant, indicating that the alteration in Ca2+ signaling induced by the deletion of RGS4 is insufficient to increase amylase secretion.
Our study provides insights into the function of RGS4 in pancreatic acinar cells. We believe that these data will help in the understanding of the function of Ca2+ dynamics and the physiology of the pancreas.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download