Abstract
To understand the roles of purinergic receptors and cellular molecules below the receptors in the vascular inflammatory response, we determined if extracellular nucleotides up-regulated chemokine expression in vascular smooth muscle cells (VSMCs). Human aortic smooth muscle cells (AoSMCs) abundantly express P2Y1, P2Y6, and P2Y11 receptors, which all respond to extracellular nucleotides. Exposure of human AoSMCs to NAD+, an agonist of the human P2Y11 receptor, and NADP+ as well as ATP, an agonist for P2Y1 and P2Y11 receptors, caused increase in chemokine (C-C motif) ligand 2 gene (CCL2) transcript and CCL2 release; however, UPT did not affect CCL2 expression. CCL2 release by NAD+ and NADP+ was inhibited by a concentration dependent manner by suramin, an antagonist of P2-purinergic receptors. NAD+ and NADP+ activated protein kinase C and enhanced phosphorylation of mitogen-activated protein kinases and Akt. NAD+- and NADP+-mediated CCL2 release was significantly attenuated by SP6001250, U0126, LY294002, Akt inhibitor IV, RO318220, GF109203X, and diphenyleneiodium chloride. These results indicate that extracellular nucleotides can promote the proinflammatory VSMC phenotype by up-regulating CCL2 expression, and that multiple cellular elements, including phosphatidylinositol 3-kinase, Akt, protein kinase C, and mitogen-activated protein kinases, are involved in that process.
Go to : 
References
1. Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, Gullestad L, Damås JK. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008; 28:1909–1919.

2. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006; 6:508–519.

3. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009; 29:313–326.

4. Ohtsuki K, Hayase M, Akashi K, Kopiwoda S, Strauss HW. Detection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: an autoradiographic study. Circulation. 2001; 104:203–208.
5. Takeya M, Yoshimura T, Leonard EJ, Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993; 24:534–539.

6. Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998; 101:353–363.

7. Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipo-protein B. J Clin Invest. 1999; 103:773–778.

8. Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997; 36:1127–1139.

9. Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors. Cell Signal. 2000; 12:351–360.
10. Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009; 29:63–72.

11. Dawicki DD, Chatterjee D, Wyche J, Rounds S. Extracellular ATP and adenosine cause apoptosis of pulmonary artery endothelial cells. Am J Physiol. 1997; 273:L485–494.

12. Malam-Souley R, Seye C, Gadeau AP, Loirand G, Pillois X, Campan M, Pacaud P, Desgranges C. Nucleotide receptor P2u partially mediates ATP-induced cell cycle progression of aortic smooth muscle cells. J Cell Physiol. 1996; 166:57–65.

13. Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991; 112:279–288.

14. Choi KH, Park JW, Kim HY, Kim YH, Kim SM, Son YH, Park YC, Eo SK, Kim K. Cellular factors involved in CXCL8 expression induced by glycated serum albumin in vascular smooth muscle cells. Atherosclerosis. 2010; 209:58–65.

15. Wang L, Karlsson L, Moses S, Hultgårdh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002; 40:841–853.

16. Sung SC, Kim K, Lee KA, Choi KH, Kim SM, Son YH, Moon YS, Eo SK, Rhim BY. 7-Ketocholesterol upregulates interleukin-6 via mechanisms that are distinct from those of tumor necrosis factor-alpha, in vascular smooth muscle cells. J Vasc Res. 2009; 46:36–44.

17. Chung SW, Park JW, Lee SA, Eo SK, Kim K. Thrombin promotes proinflammatory phenotype in human vascular smooth muscle cell. Biochem Biophys Res Commun. 2010; 396:748–754.

18. Harper S, Webb TE, Charlton SJ, Ng LL, Boarder MR. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br J Pharmacol. 1998; 124:703–710.

19. Wilden PA, Agazie YM, Kaufman R, Halenda SP. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol. 1998; 275:H1209–1215.
20. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002; 12:9–18.

21. Hou M, Möller S, Edvinsson L, Erlinge D. Cytokines induce upregulation of vascular P2Y(2) receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000; 20:2064–2069.
22. Yu SM, Chen SF, Lau YT, Yang CM, Chen JC. Mechanism of extracellular ATP-induced proliferation of vascular smooth muscle cells. Mol Pharmacol. 1996; 50:1000–1009.
23. Needleman P, Minkes MS, Douglas JR Jr. Stimulation of prostaglandin biosynthesis by adenine nucleotides. Profile of prostaglandin release by perfused organs. Circ Res. 1974; 34:455–460.
24. Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003; 42:1075–1081.
Go to : 
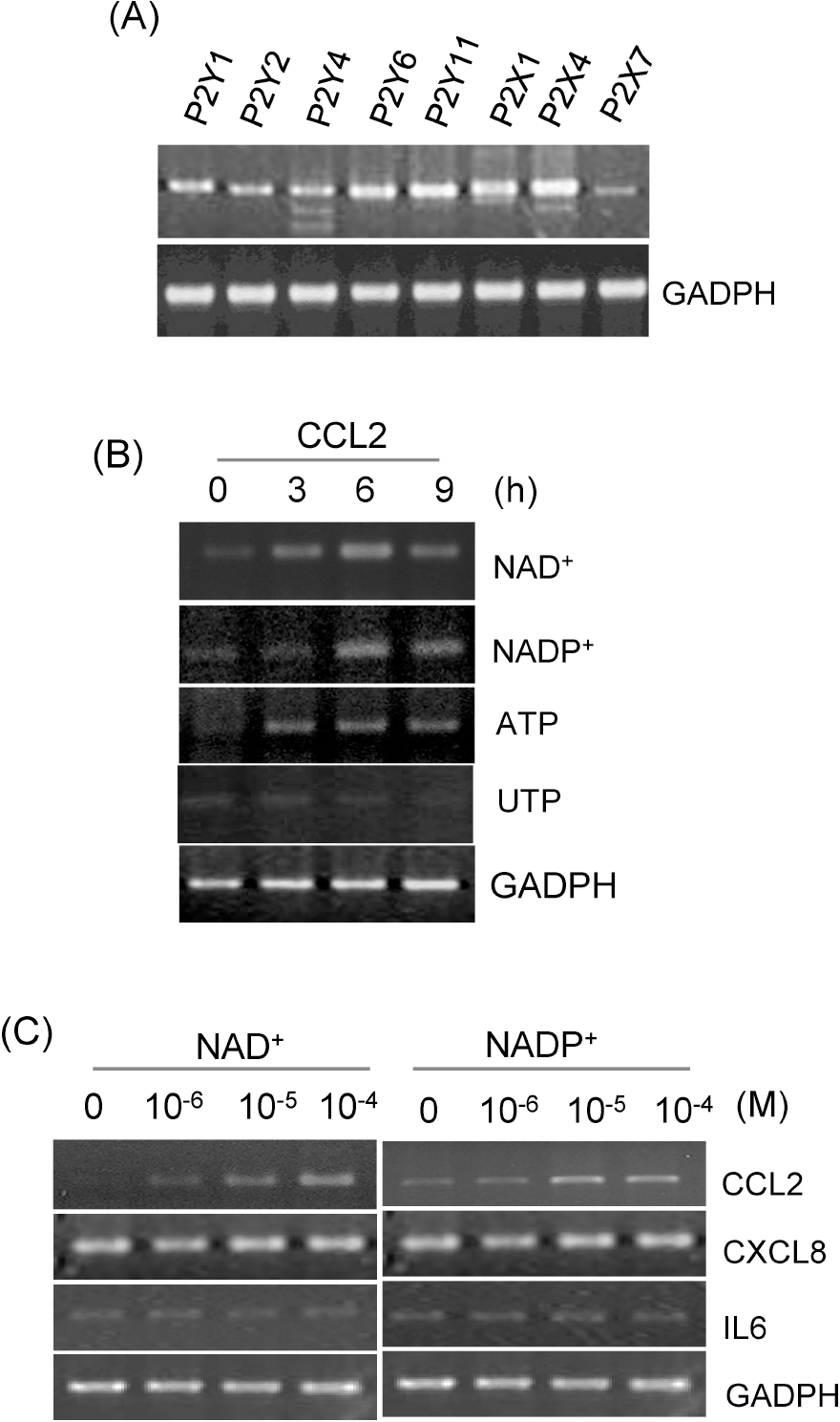 | Fig. 1.The effects of extracellular nucleotides on CCL2 gene transcripts in human VSMCs. (A) Total RNA was isolated from human AoSMCs, and transcripts of the indicated P2 purinergic receptors were identified by RT-PCR. (B) Human AoSMCs were treated with nucleotides for the indicated time periods, and CCL2 transcripts were amplified by RT-PCR. (C) Human AoSMCs were treated for 6 h with the indicated concentrations of NAD+ or NADP+, and induction of CCL2 gene transcripts was examined by RT-PCR. |
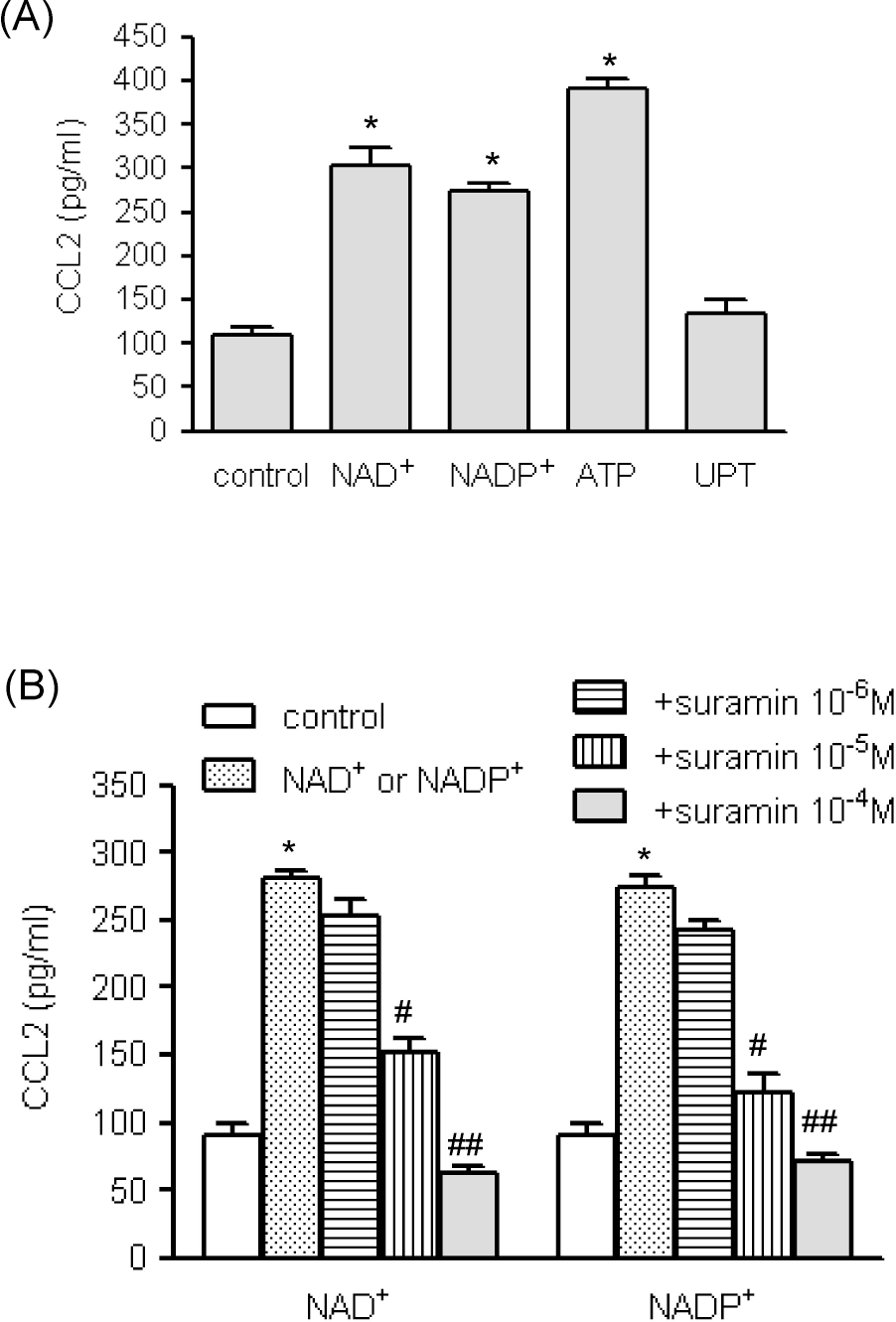 | Fig. 2.The effects of nucleotides on NAD+- and NADP+-mediated CCL2 release. (A) Human AoSMCs (1×106 cells) were incubated in the absence (control) or presence of indicated nucleotides (10–4 M for each nucleotide). Culture medium was collected and the amount of secreted CCL2 was measured by ELISA. ∗p<0.001 vs. control. (B). Human AoSMCs were incubated with NAD+ or NADP+ (10–4 M) in the absence or presence of indicated concentrations of suramin. CCL2 released into the medium was measured by ELISA. ∗p<0.001 vs. control; #p<0.01 vs. NAD+ or NADP+, ##p<0.001 vs. NAD+ or NADP+. |
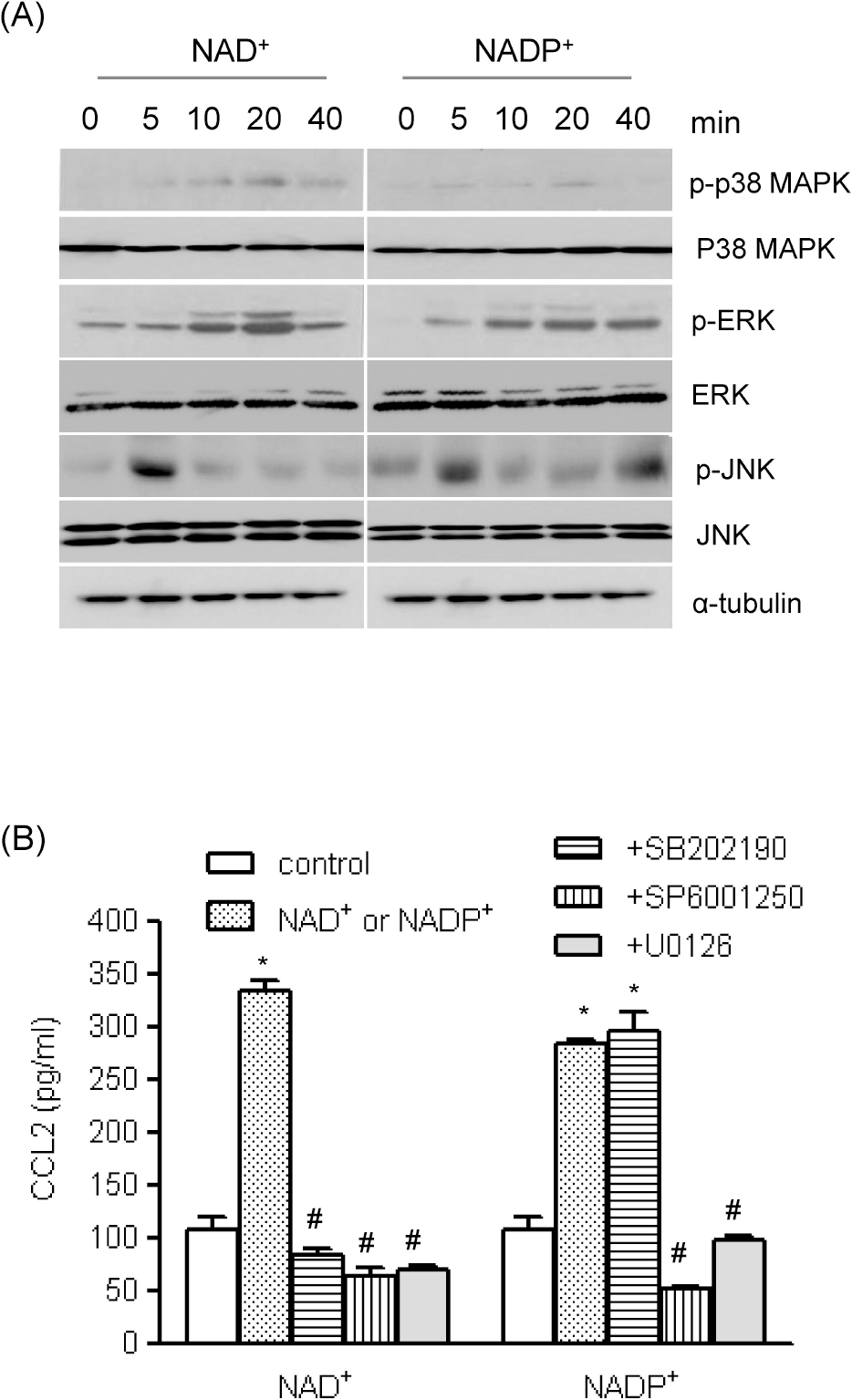 | Fig. 3.The roles of MAPKs in NAD+- and NADP+-mediated CCL2 release. (A) Human AoSMCs were exposed to NAD+ or NADP+ for the indicated time periods, after which an equal amount of protein was subjected to Western blot analysis using antibodies for α-tubulin and phosphorylated and unphosphorylated forms of ERK, p38 MAPK, and JNK. (B) Human AoSMCs were incubated for an hour with SP600125, U0126, and SB202190 (10 μM each) and stimulated with NAD+ or NADP+. Culture media were collected to measure the amount of secreted CCL2. ∗p< 0.001 vs. control, #p<0.001 vs. NAD+ or NADP+. |
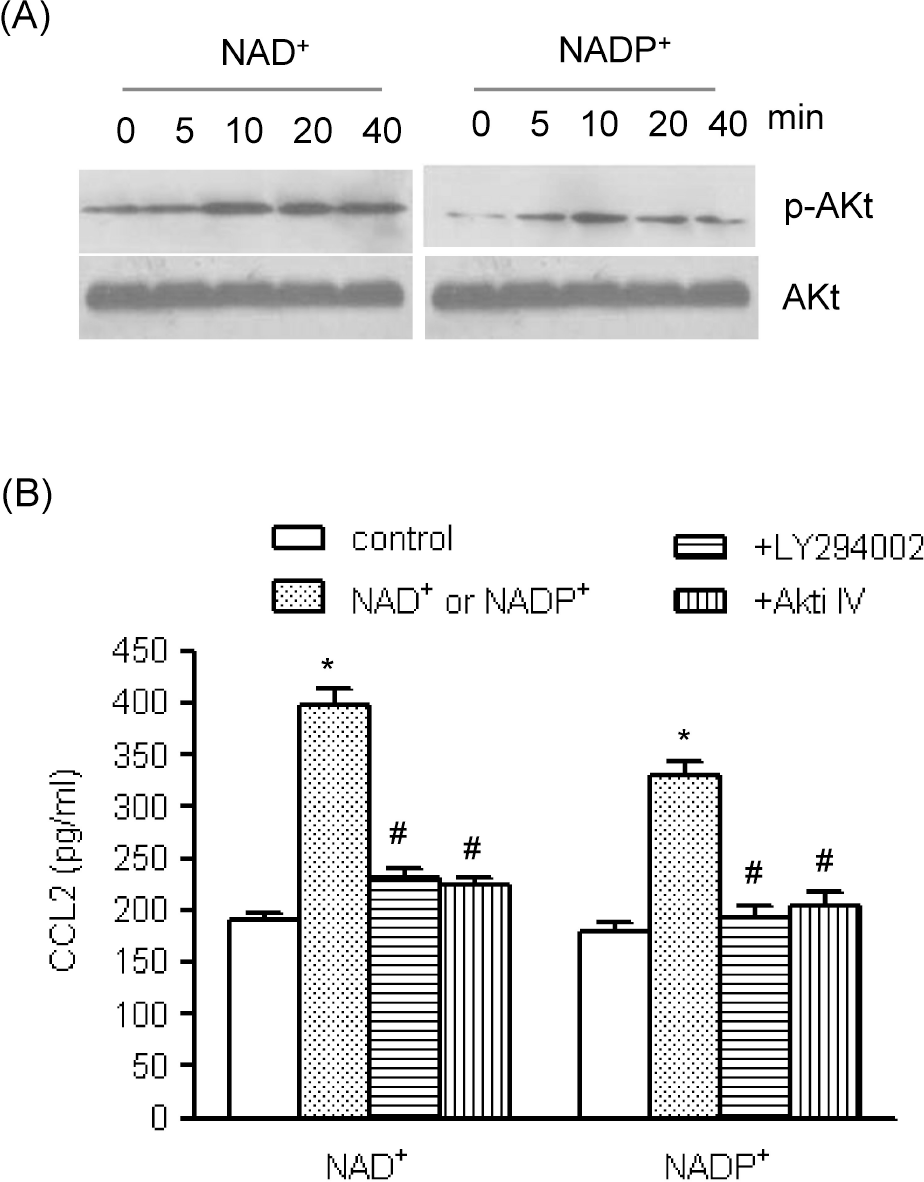 | Fig. 4.The roles of Akt pathways in NAD+- and NADP+-mediated CCL2 release. (A) Human AoSMCs were exposed to NAD+ or NADP+ for the indicated time periods, after which an equal amount of protein was subjected to Western blot analysis using antibodies for Akt and phosphorylated Akt. (B) Human AoSMCs were incubated for 1 h with LY294002 and Akti IV (10 μM each) and stimulated with NAD+ or NADP+. The amount of secreted CCL2 was measured by ELISA. ∗p<0.001 vs. control, #p<0.001 vs. NAD+ or NADP+. |
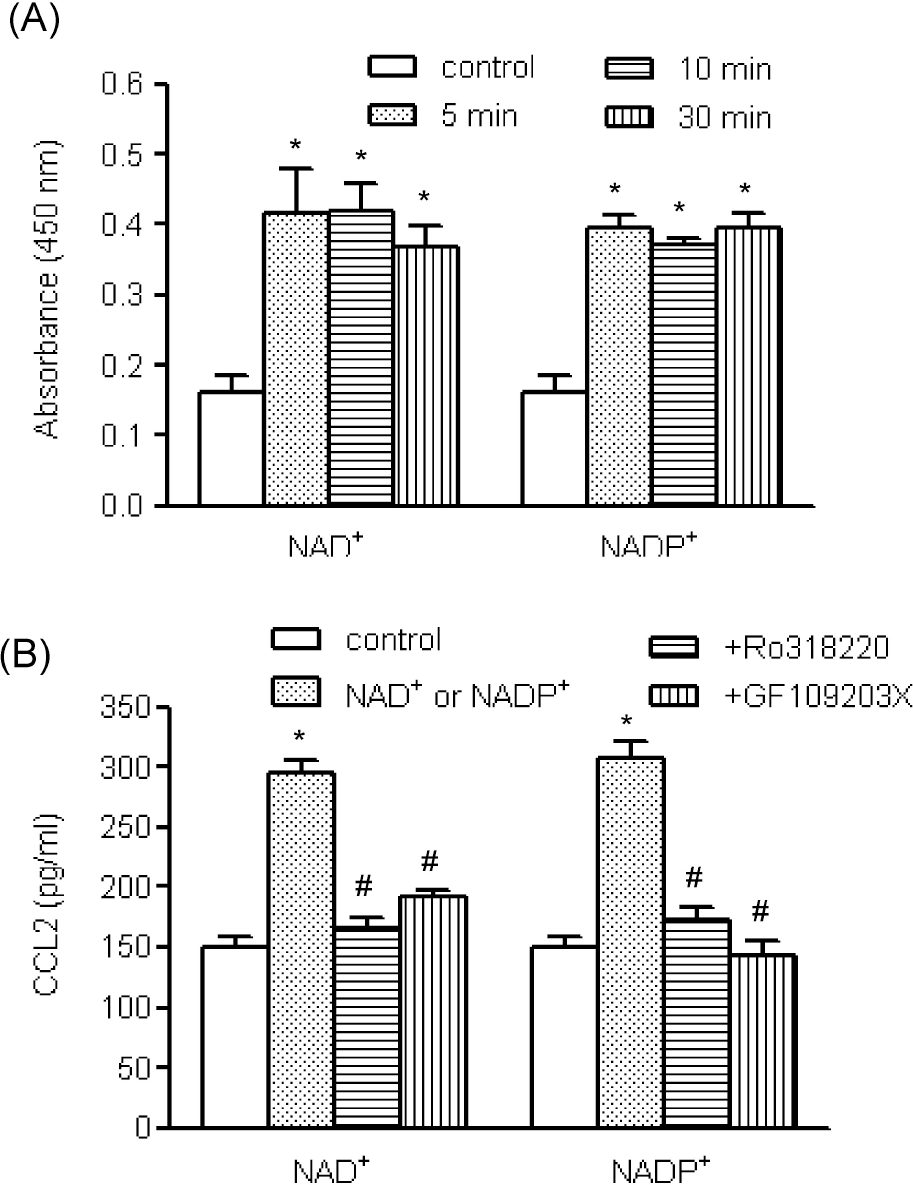 | Fig. 5.The roles of PKC in NAD+- and NADP+-mediated CCL2 release. (A) Human AoSMCs were exposed to NAD+ or NADP+ for the indicated time periods, after which PKC activity was determined. ∗p<0.01 vs. control. (B) Human AoSMCs were incubated for 1 h with GF109203X (3 μM) and RO318220 (1 μM) and stimulated with NAD+ or NADP+. The amount of secreted CCL2 was measured by ELISA. ∗p<0.001 vs. control, #p<0.001 vs. NAD+ or NADP+. |
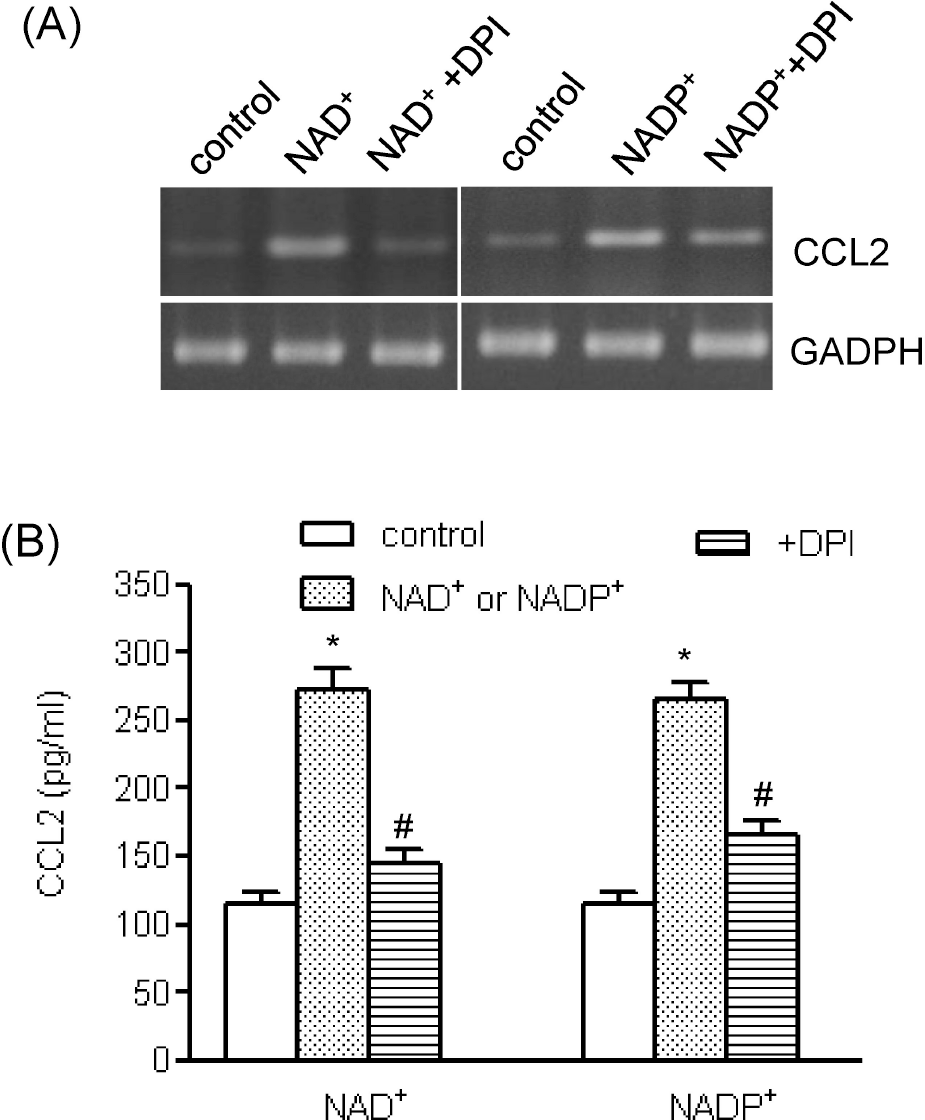 | Fig. 6.The roles of NADPH oxidase in NAD+- and NADP+ –mediated CCL2 release. Human AoSMCs were incubated for 1 h with DPI (10 μM) and stimulated with NAD+ or NADP+. CCL2 gene transcript was amplified by RT-PCR (A), and the amount of released CCL2 was measured by ELISA (B). ∗p<0.001 vs. control, #p<0.001 vs. NAD+ or NADP+. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download