Abstract
Shilajit, a medicine herb commonly used in Ayurveda, has been reported to contain at least 85 minerals in ionic form that act on a variety of chemical, biological, and physical stressors. The substantia gelatinosa (SG) neurons of the trigeminal subnucleus caudalis (Vc) are involved in orofacial nociceptive processing. Shilajit has been reported to be an injury and muscular pain reliever but there have been few functional studies of the effect of Shilajit on the SG neurons of the Vc. Therefore, whole cell and gramicidin-perfotrated patch clamp studies were performed to examine the action mechanism of Shilajit on the SG neurons of Vc from mouse brainstem slices. In the whole cell patch clamp mode, Shilajit induced short-lived and repeatable inward currents under the condition of a high chloride pipette solution on all the SG neurons tested. The Shilajit-induced inward currents were concentration dependent and maintained in the presence of tetrodotoxin (TTX), a voltage gated Na+ channel blocker, CNQX, a non-NMDA glutamate receptor antagonist, and AP5, an NMDA receptor antagonist. The Shilajit-induced responses were partially suppressed by picrotoxin, a GABAA receptor antagonist, and totally blocked in the presence of strychnine, a glycine receptor antagonist, however not affected by mecamylam ine hydrochloride (MCH), a nicotinic acetylcholine receptor antagonist. Under the potassium gluconate pipette solution at holding potential 0 mV, Shilajit induced repeatable outward current. These results show that Shilajit has inhibitory effects on the SG neurons of Vc through chloride ion channels by activation of the glycine receptor and GABAA receptor, indicating that Shilajit contains sedating ingredients for the central nervous system. These results also suggest that Shilajit may be a potential target for modulating orofacial pain processing.
Go to : 
References
1. Ghosal S. Chemistry of shilajit, an immunomodulatory Ayurvedic Rasayana. Pure and Appl Chem. 1990; 62:1285–1288.
2. Ali M, Sahrawat I, Singh O. Phytochemical investigation of Shilajit. India J Chem. 2004; 43B:2217–2222.

3. Garedew A, Feist M, Schmolz E, Lamprecht I. Thermal analysis of mumiyo, the legendary folk remedy from the Himalaya region. Thermochimica Acta. 2004; 417:301–309.

4. Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: a review. Phytother Res. 2007; 21:401–405.

5. Jaiswal AK, Bhattacharya SK. Effects of Shilajit on memory, anxiety and brain monoamines in rats. Indian Journal of Pharmacology. 1992; 24:12–17.
6. Ghosal S, Singh SK, Srivastava RS. Shilajit part 2. Biphenyl metabolites from Trifolium repens. J Chem Res. 1988; 196:165–166.
7. Charaka S, Sharma PV. Karaprchitiya RasayanaPada. 4th ed.Chowkhambha orientalia Chikitsasthana;Varanasi: 1998. p. 49–50.
8. Schepetkin I, Khlebnikov A, Kwon BS. Medical drugs from humus matter: focus on mumie. Drug Dev Res. 2002; 57:140–159.

9. Sessle BJ. Mechanisms of trigeminal and occipital pain. Pain Rev. 1996; 3:91–116.
10. Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis). J Comp Neurol. 1978; 180:395–413.

11. Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979; 186:117–131.

12. Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V). J Comp Neurol. 1988; 267:172–189.

13. Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004; 91:2413–2421.

14. Acharya SB, Frotan MH, Goel RK, Tripathi SK, Das PK. Pharmacological actions of Shilajit. Indian J Exp Biol. 1988; 26:775–777.
15. Tiwari P, Ramarao P, Ghosal S. Effects of Shilajit on the development of tolerance to morphine in mice. Phytother Res. 2001; 15:177–179.

16. Kuffler SW, Edwards C. Mechanism of gamma aminobutyric acid (GABA) action and its relation to synaptic inhibition. J Neurophysiol. 1958; 21:589–610.

17. Takeuchi A, Onodera K. Effect of bicuculline on the GABA receptor of the crayfish neuromuscular junction. Nat New Biol. 1972; 236:55–56.

18. Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M. (–)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980; 283:92–94.

19. Lee J, Back SK, Lim EJ, Cho GC, Kim MA, Kim HJ, Lee MH, Na HS. Are spinal GABAergic elements related to the manifestation of neuropathic pain in rat? Korean J Physiol Pharmacol. 2010; 14:59–69.

20. Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol. 1995; 484:77–86.

22. Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004; 84:1051–1095.

23. Bradaïa A, Trouslard J. Nicotinic receptors regulate the release of glycine onto lamina X neurones of the rat spinal cord. Neuropharmacology. 2002; 43:1044–1054.

24. Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987; 385:243–286.

25. Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972; 274:313–322.
26. Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995; 57:27–35.

Go to : 
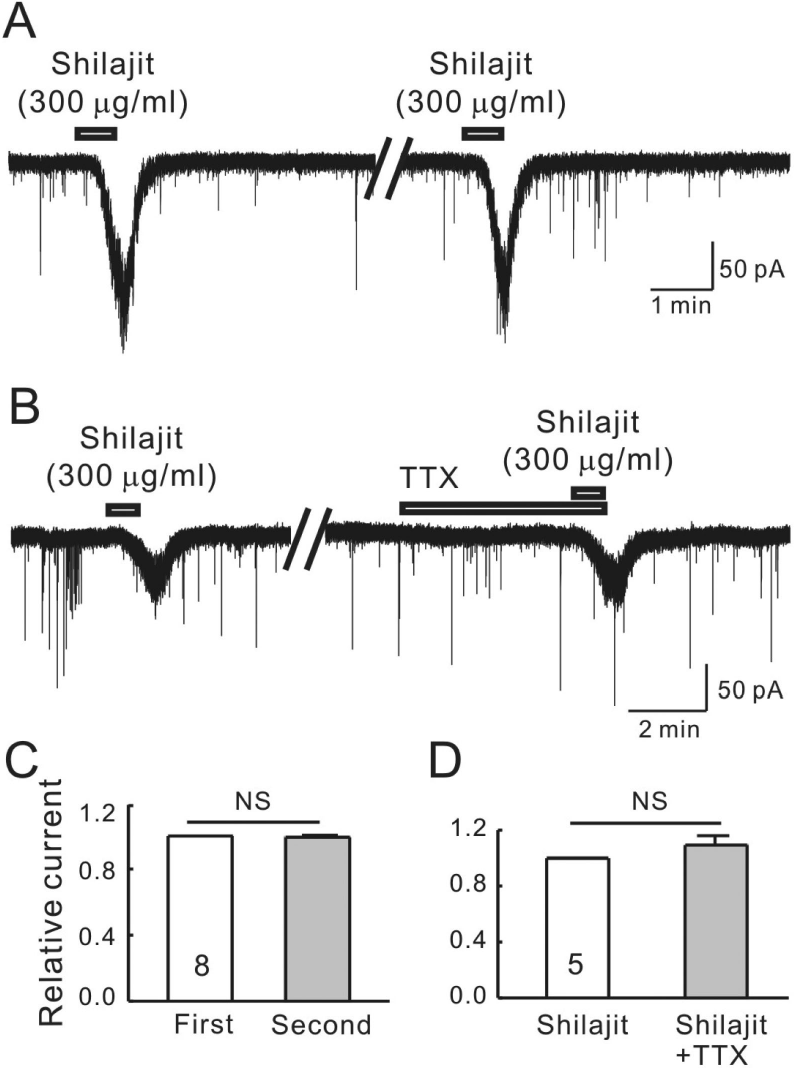 | Fig. 1.Shilajit-induced currents are repeatable and mediated by postsynaptic cell actions on SG neurons. (A) A representative trace showing the repeatable inward currents induced by Shilajit (300μ g/ml). (B) A representative trace showing the inward current induced by Shilajit (300μg/ml) alone and in the presence of TTX (0.5μM). (C) Relative bars showing a comparison of the membrane current changes by the repeated application of Shilajit (300μg/ml, n=8, paired t-test). (D) Relative bars showing a comparison of the membrane current changes by the Shilajit alone and in the presence of TTX (300μg/ml, n=5, paired t-test). |
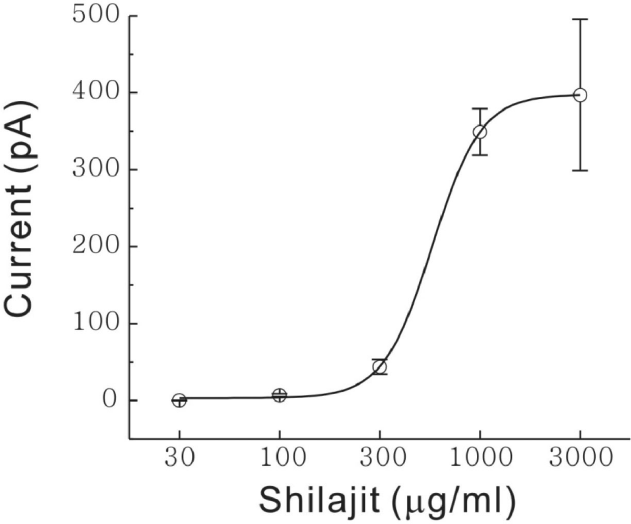 | Fig. 2.Shilajit-induced concentration dependent responses on SG neurons. Curve figure showing the response of 30, 100, 300μg/ml and 1, 3 mg/ml Shilajit (n=8). EC50 was estimated to 562μg/ml. |
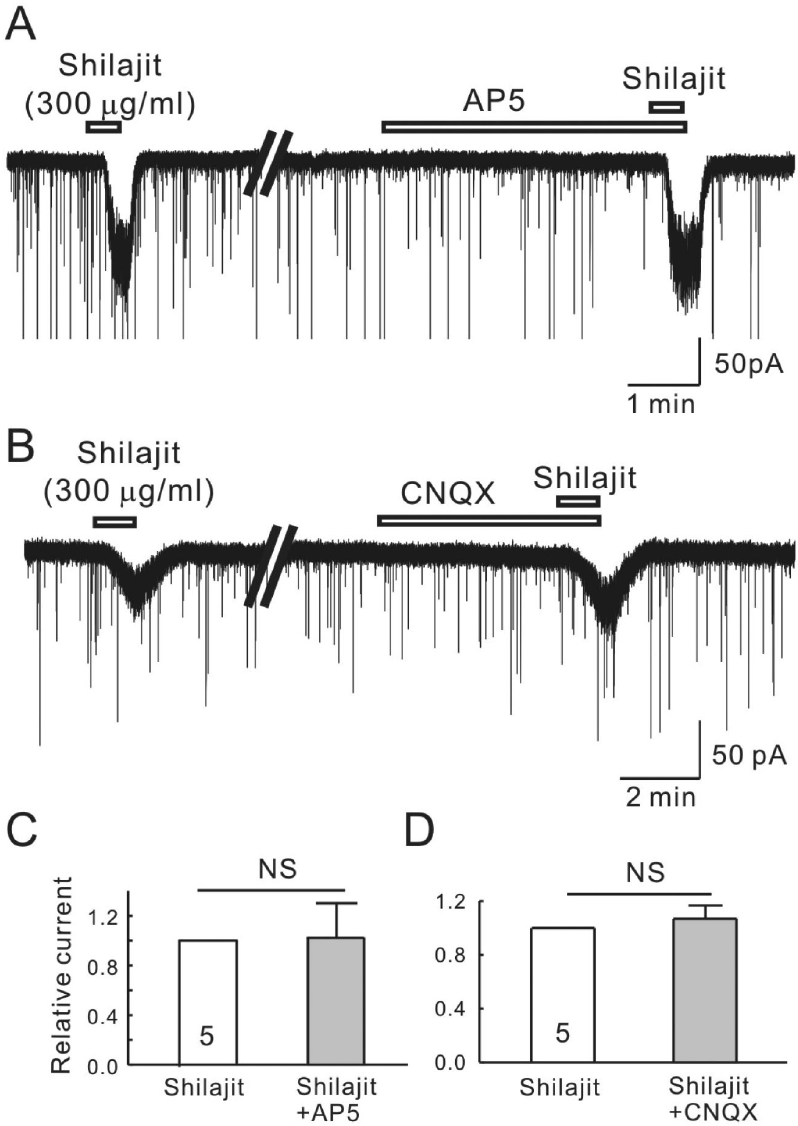 | Fig. 3.Shilajit-activated currents were not mediated by the NMDA and non-NMDA glutamate receptors on the SG neurons. (A, B) Representative traces showing the inward current induced by Shilajit application (300μg/ml). The Shilajit induced inward current persisted in the presence AP5 (NMDA receptor antagonist) and CNQX (non-NMDA receptor antagonist). (C, D) Relative responses of Shilajit in the presence of AP5 or CNQX compared to Shilajit alone (n=5). |
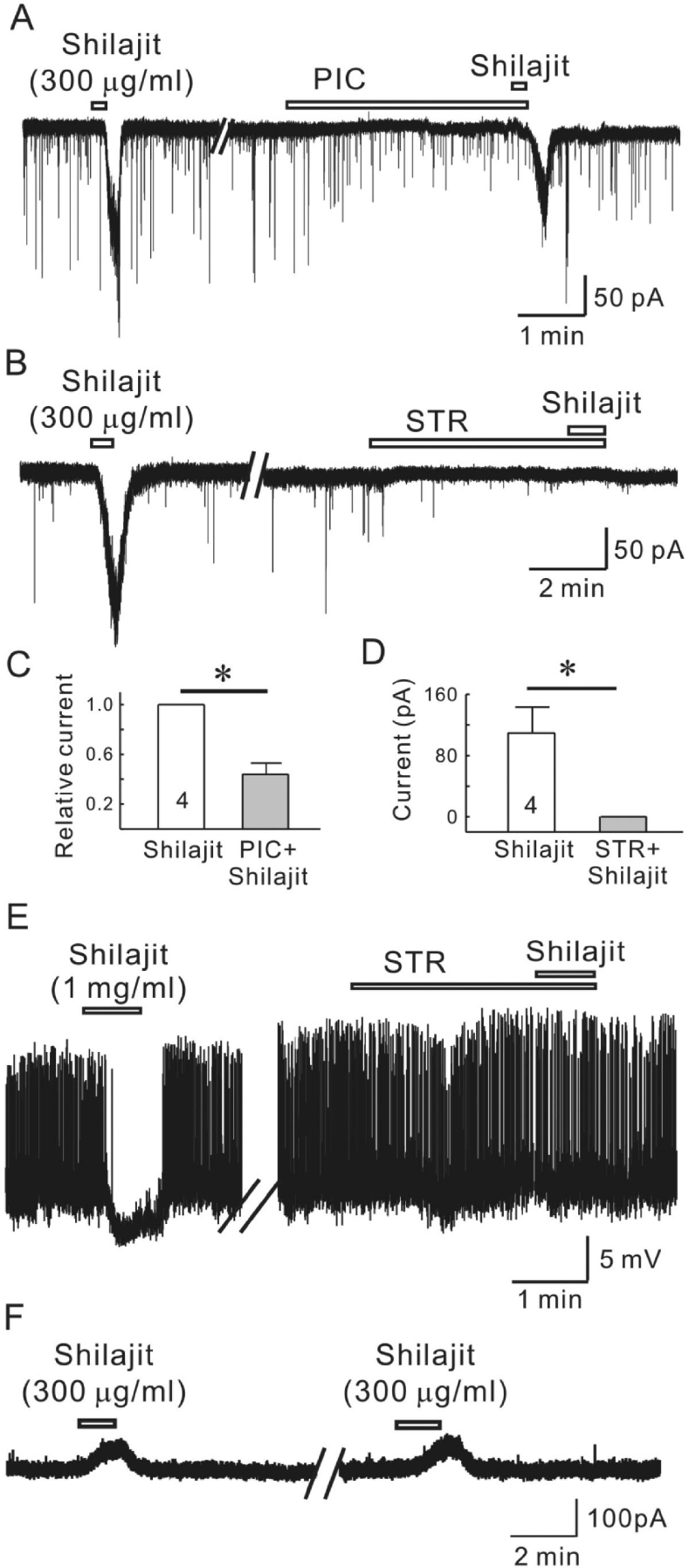 | Fig. 4.Silajit-induced inward currents are mediated by the activation of glycine and GABAA receptors. (A) Representative trace showing the inward current induced by Shilajit (300μg/ml). Shilajit-induced inward current was reduced in the presence of picrotoxin (PIC, GABAA receptor antagonist). (B) A representative trace showing the inward current induced by Shilajit application (300μg/ml) in whole-cell recording. The Shilajit-induced inward current was reduced in the presence of strychnine. (C) Relative response of Shilajit in the presence of PIC compared to Shilajit alone (n=4), ∗p<0.05. (D) Membrane current change in Shilajit alone and in the presence of strychnine (STR, a glycine receptor antagonist, n=4) ∗p<0.05. (E) A representative trace showing hyperpolarization induced by Shilajit application (1 mg/ml) in gramicidin-perforated recording (RMP=–54 mV). Shilajit-induced hyperpoalrization was blocked in the presence of strychnine. Membrane potential change in Shilajit alone and in the presence of strychnine (STR, n=3) ∗p<0.05. (F) A representative trace showing the repeatable outward currents induced by Shilajit (300μg/ml) under potassium gluconate pipette solution at VH=0 mV. |
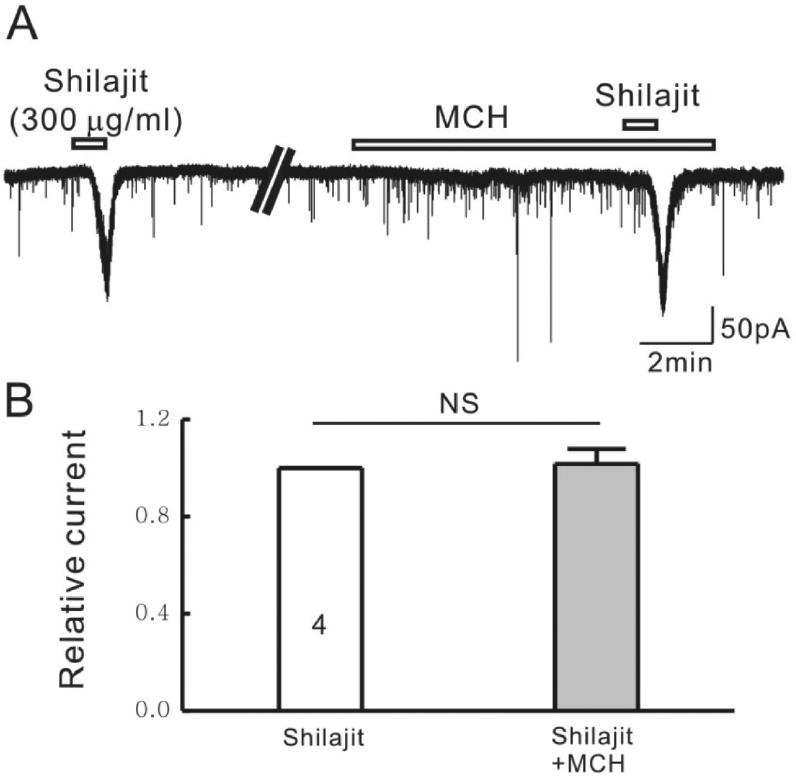 | Fig. 5.Silajit-induced inward currents are not mediated by the activation of nACh receptors. (A) Representative trace showing the inward current induced by Shilajit (300μg/ml). Shilajit-induced inward current was not affected in the presence of MCH (nACh receptor antagonist). (B) Relative response of Shilajit in the presence of MCH compared to Shilajit alone (n=4), ∗p<0.05. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download