Abstract
Nitric oxide (NO) and atrial natriuretic peptide (ANP) may induce vascular relaxation by increasing the production of cyclic guanosine monophosphate (cGMP), an important mediator of vascular tone during sepsis. This study aimed to determine whether regulation of NO and the ANP system is altered in lipopolysaccharide (LPS)-induced kidney injury. LPS (10 mg · kg–1) was injected in the tail veins of male Sprague-Dawley rats; 12 hours later, the kidneys were removed. Protein expression of NO synthase (NOS) and neutral endopeptidase (NEP) was determined by semiquantitative immunoblotting. As an index of synthesis of NO, its stable metabolites (nitrite/nitrate, NOx) were measured using colorimetric assays. mRNA expression of the ANP system was determined by real-time polymerase chain reaction. To determine the activity of guanylyl cyclase (GC), the amount of cGMP generated in response to sodium nitroprusside (SNP) and ANP was calculated. Creatinine clearance decreased and fractional excretion of sodium increased in LPS-treated rats compared with the controls. Inducible NOS protein expression increased in LPS-treated rats, while that of endothelial NOS, neuronal NOS, and NEP remained unchanged. Additionally, urinary and plasma NOx levels increased in LPS-treated rats. SNP-stimulated GC activity remained unchanged in the glomerulus and papilla in the LPS-treated rats. mRNA expression of natriuretic peptide receptor (NPR)-C decreased in LPS-treated rats, while that of ANP and NPR-A did not change. ANP-stimulated GC activity reduced in the glomerulus and papilla. In conclusion, enhancement of the NO/cGMP pathway and decrease in ANP clearance were found play a role in the pathogenesis of LPS-induced kidney injury.
Go to : 
References
1. Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990; 113:227–242.
3. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997; 15:323–350.

5. Carnio EC, Stabile AM, Batalhão ME, Silva JS, Antunes-Rodrigues J, Branco LG, Magder S. Vasopressin release during endotoxaemic shock in mice lacking inducible nitric oxide synthase. Pflugers Arch. 2005; 450:390–394.

6. Knowles RG, Moncada S. Nitric oxide as a signal in blood vessels. Trends Biochem Sci. 1992; 17:399–402.

7. Scicluna JK, Mansart A, Ross JJ, Reilly CS, Brown NJ, Brookes ZL. Lipopolysaccharide alters vasodilation to atrial natriuretic peptide via nitric oxide and endothelin-1: time-dependent effects. Eur J Pharmacol. 2009; 621:67–70.

8. De Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981; 28:89–94.

9. Totsune K, Takahashi K, Murakami O, Satoh F, Sone M, Saito T, Sasano H, Mouri T, Abe K. Natriuretic peptides in the human kidney. Hypertension. 1994; 24:758–762.

10. Winquist RJ, Faison EP, Waldman SA, Schwartz K, Murad F, Rapoport RM. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci USA. 1984; 81:7661–7664.

11. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
12. Chinkers M, Garbers DL. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989; 245:1392–1394.

13. Zhang C, Walker LM, Mayeux PR. Role of nitric oxide in lipopolysaccharide-induced oxidant stress in the rat kidney. Biochem Pharmacol. 2000; 59:203–209.

14. Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, Poole BD, Bowler RP, Day BJ, Crapo JD, Schrier RW. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol. 2003; 284:F532–537.

15. Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol. 1995; 268:F885–898.

16. Markewitz BA, Michael JR, Kohan DE. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest. 1993; 91:2138–2143.

18. Del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000; 10:95–112.
19. Noiri E, Peresleni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest. 1996; 97:2377–2383.

20. Aiura K, Ueda M, Endo M, Kitajima M. Circulating concentrations and physiologic role of atrial natriuretic peptide during endotoxic shock in the rat. Crit Care Med. 1995; 23:1898–1906.

Go to : 
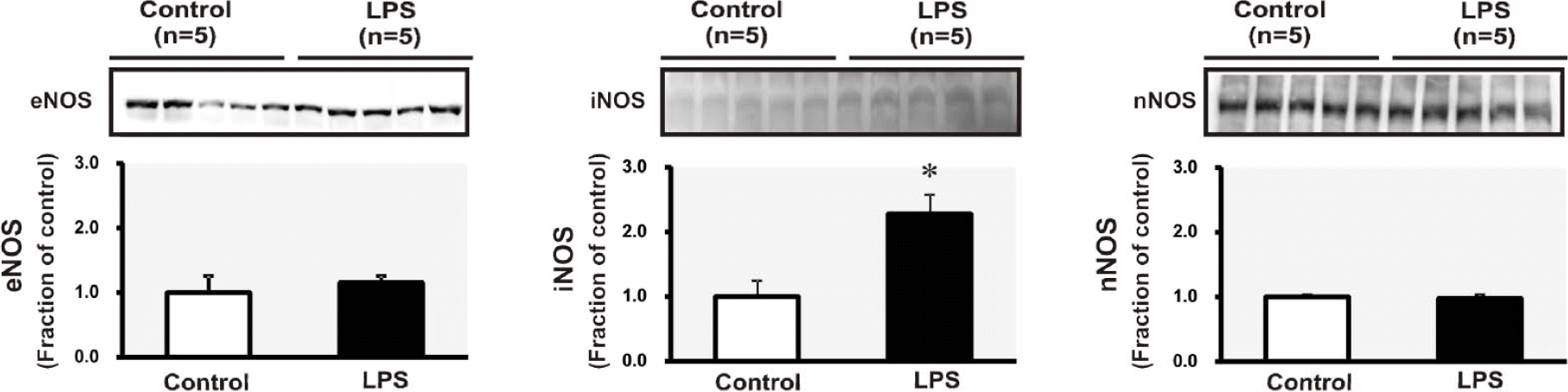 | Fig. 1.Semiquantitative immunoblotting of endothelial nitric oxide synthase (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS) in the kidney. ∗p<0.05 compared with control. |
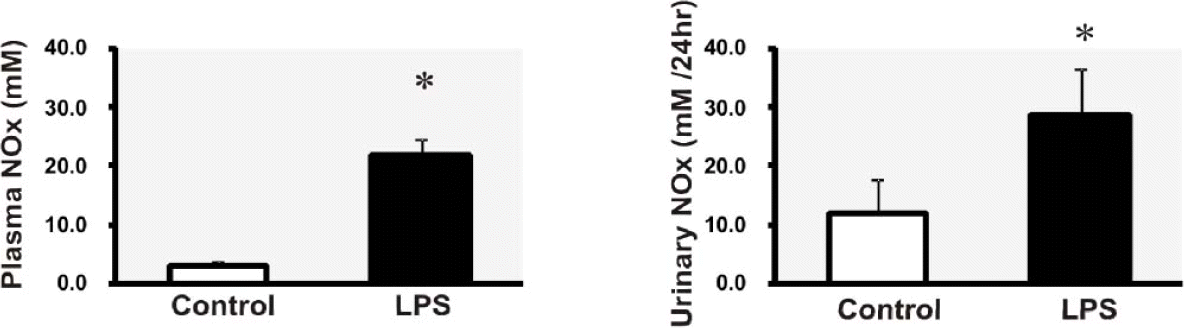 | Fig. 2.Nitric oxide metabolites (nitrite/nitrate, NOx) in plasma and urine. ∗p<0.05 compared with control. |
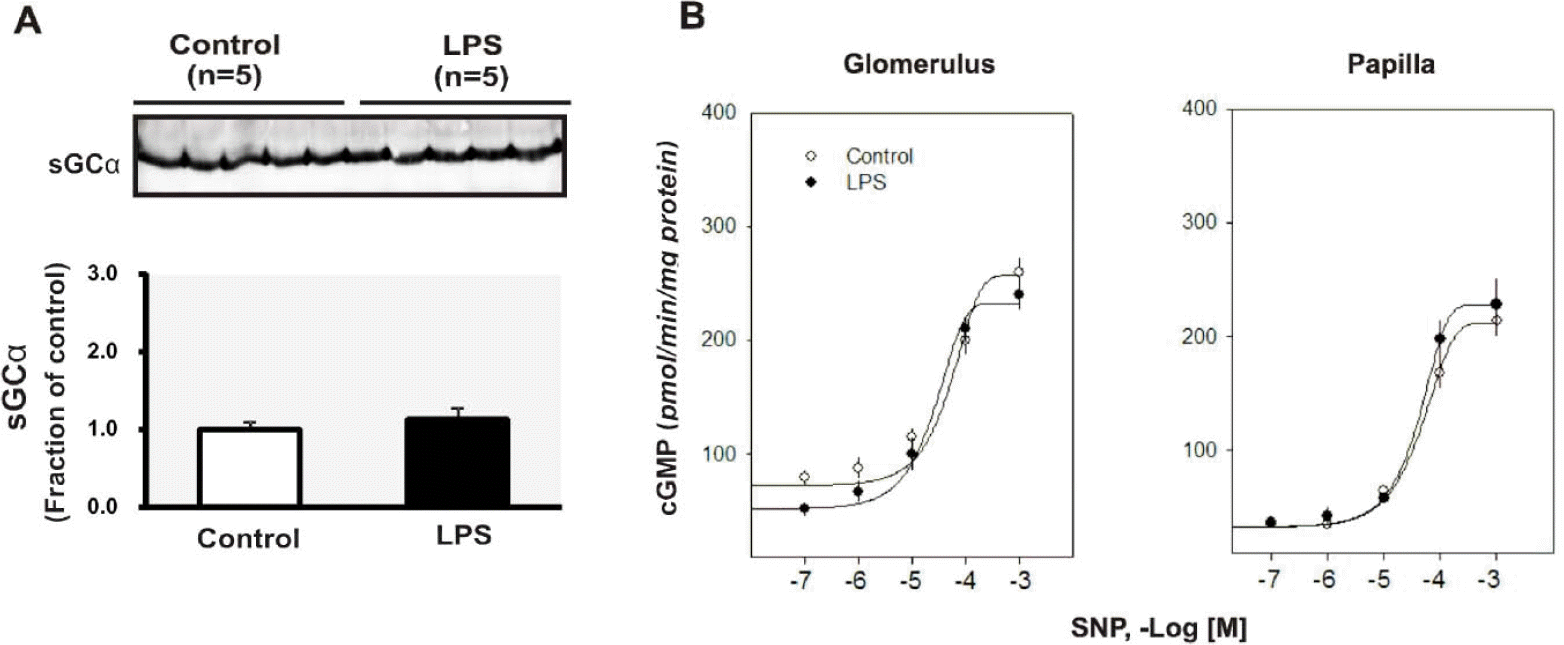 | Fig. 3.(A) Semiquantitative immunoblotting of soluble guanylnyl cyclase (sGC) in the kidney. (B) cGMP production in response to sodium nitroprusside (SNP) in the glomerulus and papilla. Each point represents mean±SEM of experimental rats. ∗p<0.05 compared with control. |
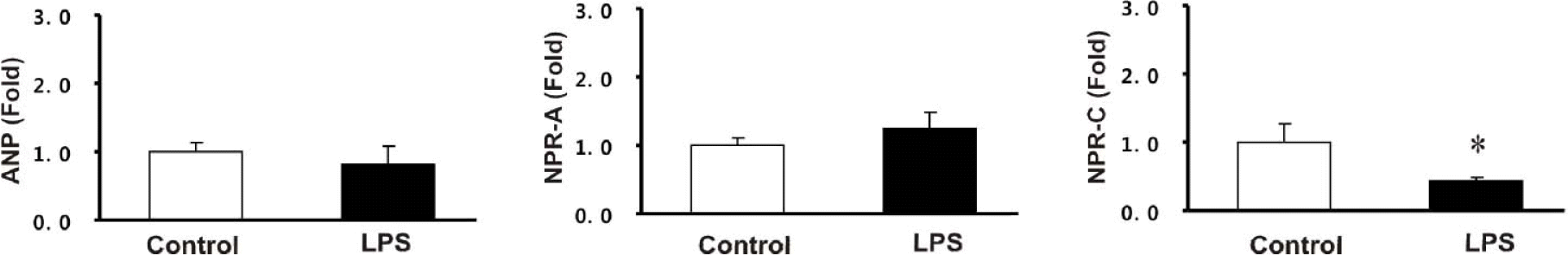 | Fig. 4.The mRNA expression of atrial natriuretic peptide (ANP), natriuretic peptide receptor type A and C (NPR-A, NPR-C) in whole kidney. Columns show real-time PCR data representing control and LPS groups. ∗p<0.05 compared with control. |
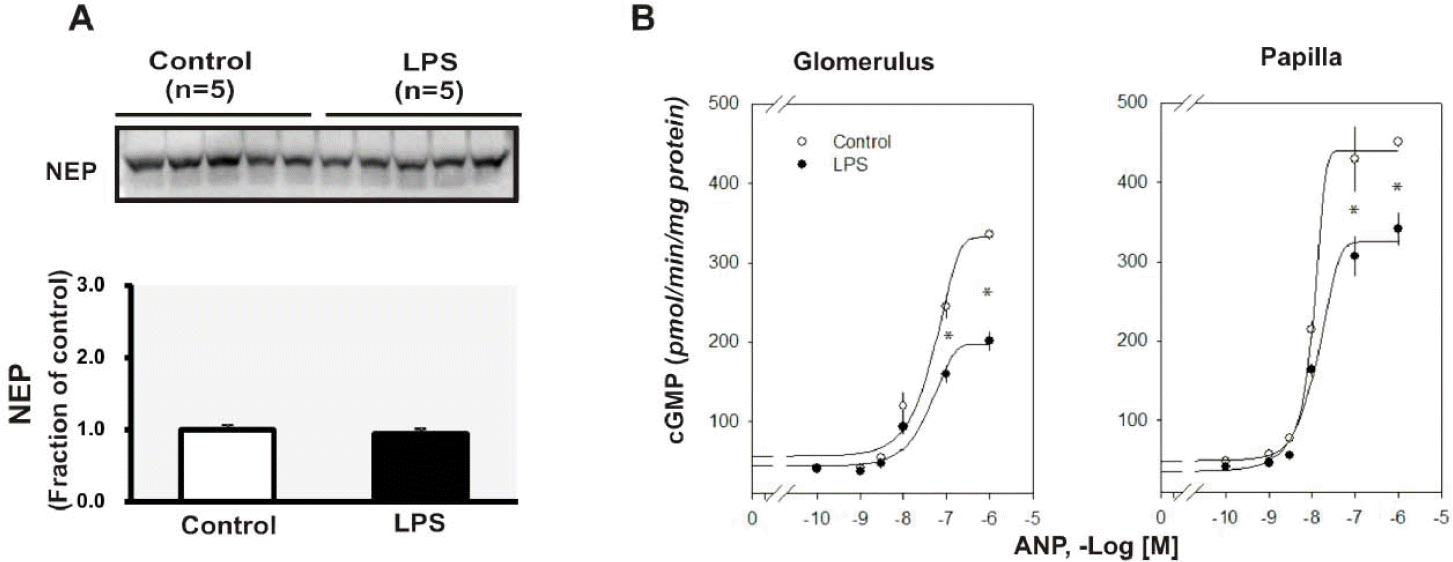 | Fig. 5.(A) Semiquantitative immunoblotting of neutral endopeptidase (NEP) in the kidney. (B) cGMP production in response to atrial natriuretic peptide (ANP) in the glomerulus and papilla. Each point represents mean±SEM of experimental rats. ∗p<0.05 compared with control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download