Abstract
The aim of this study was to evaluate the preventive role of epigallocatechin-3 gallate (EGCG, a derivative of green tea) in ischemia/reperfusion (I/R) injury of isolated rat hearts. It has been suggested that EGCG has beneficial health effects, including prevention of cancer and heart disease, and it is also a potent antioxidant. Rat hearts were subjected to 20 min of normoxia, 20 min of zero-flow ischemia and then 50 min of reperfusion. EGCG was perfused 10 min before ischemia and during the whole reperfusion period. EGCG significantly increased left ventricular developed pressure (LVDP) and increased maximum positive and negative dP/dt (+/-dP/dtmax). EGCG also significantly increased the coronary flow (CF) at baseline before ischemia and at the onset of the reperfusion period. Moreover, EGCG decreased left ventricular end diastolic pressure (LVEDP). This study showed that lipid peroxydation was inhibited and Mn-SOD and catalase expressions were increased in the presence of EGCG. In addition, EGCG increased levels of Bcl-2, Mn-superoxide dismutase (SOD), and catalase expression and decreased levels of Bax and increased the ratio of Bcl-2/Bax in isolated rat hearts. Cleaved caspase-3 was decreased after EGCG treatment. EGCG markedly decreased the infarct size while attenuating the increase in lactate dehydrogenase (LDH) levels in the effluent. In summary, we suggest that EGCG has a protective effect on I/R-associated hemodynamic alteration and injury by acting as an antioxidant and anti-apoptotic agent in one.
Go to : 
References
1. Ostadal B. The past, the present and the future of experimental research on myocardial ischemia and protection. Pharmacol Rep. 2009; 61:3–12.

3. Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008; 88:581–609.

4. Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009; 15:RA209–219.
5. Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA. 1987; 84:1404–1407.

6. Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006; 70:181–190.

7. Näslund U, Häggmark S, Johansson G, Marklund SL, Reiz S, Oberg A. Superoxide dismutase and catalase reduce infarct size in a porcine myocardial occlusion-reperfusion model. J Mol Cell Cardiol. 1986; 18:1077–1084.
8. Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, Guyton RA, Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000; 45:651–660.

9. Zhao ZQ, Morris CD, Budde JM, Wang NP, Muraki S, Sun HY, Guyton RA. Inhibition of myocardial apoptosis reduces infarct size and improves regional contractile dysfunction during reperfusion. Cardiovasc Res. 2003; 59:132–142.

10. Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol. 2002; 13:41–49.

11. Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999; 58:911–915.

12. Peairs A, Dai R, Gan L, Shimp S, Rylander MN, Li L, Reilly CM. Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell Mol Immunol. 2010; 7:123–132.

13. Serafini M, Ghiselli A, Ferro-Luzzi A. In vivo antioxidant effect of green and black tea in man. Eur J Clin Nutr. 1996; 50:28–32.
14. Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006; 67:1849–1855.

15. Lorenz M, Hellige N, Rieder P, Kinkel HT, Trimpert C, Staudt A, Felix SB, Baumann G, Stangl K, Stangl V. Positive inotropic effects of epigallocatechin-3-gallate (EGCG) involve activation of Na+/H+ and Na+/Ca2+ exchangers. Eur J Heart Fail. 2008; 10:439–445.
16. Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004; 18:1621–1623.
17. Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M, Tomita T. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001; 131:27–32.

18. Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab. 2007; 292:E1378–1387.

19. Piper HM, Abdallah Y, Schäfer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004; 61:365–371.

20. Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998; 38:291–300.

21. Hirai M, Hotta Y, Ishikawa N, Wakida Y, Fukuzawa Y, Isobe F, Nakano A, Chiba T, Kawamura N. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci. 2007; 80:1020–1032.

22. Song DK, Jang Y, Kim JH, Chun KJ, Lee D, Xu Z. Polyphenol (–)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial K(ATP) channel activation in isolated rat hearts. J Korean Med Sci. 2010; 25:380–386.

23. Rajak S, Banerjee SK, Sood S, Dinda AK, Gupta YK, Gupta SK, Maulik SK. Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats. Phytother Res. 2004; 18:54–60.
24. Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007; 18:287–296.

25. Devika PT, Stanely Mainzen Prince P. (–)Epigallocatechingallate protects the mitochondria against the deleterious effects of lipids, calcium and adenosine triphosphate in isoproterenol induced myocardial infarcted male Wistar rats. J Appl Toxicol. 2008; 28:938–944.

26. Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011; 1813:1382–1394.

27. Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003; 284:H277–282.

28. Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998; 98:1355–1357.

29. Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004; 10:55–62.

30. Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007; 73:689–698.

31. Piao CS, Gao S, Lee GH, Kim do S, Park BH, Chae SW, Chae HJ, Kim SH. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial K(ATP) channels. Pharmacol Res. 2010; 61:342–348.

32. Dorn GW 2nd. Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009; 81:465–473.

33. Zhong H, Xin H, Wu LX, Zhu YZ. Salidroside attenuates apoptosis in ischemic cardiomyocytes: a mechanism through a mitochondria-dependent pathway. J Pharmacol Sci. 2010; 114:399–408.

34. Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ, Chae SW. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005; 70:1066–1078.

Go to : 
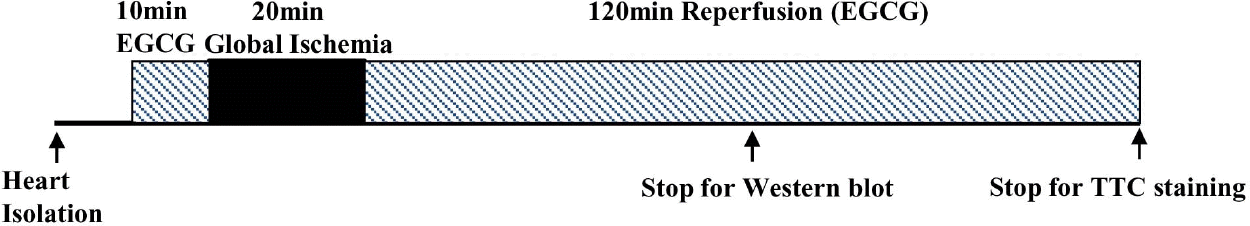 | Fig. 1.Experimental protocol. All hearts were perfused for a total of 90 min or 160 min consisting of a 20-min pre-ischemia period followed by 20 min of global ischemia and 50 min or 120 min of reperfusion at 37°C. There was no ischemia period for the sham group. EGCG was perfused 10 min before ischemia and during the whole reperfusion period. |
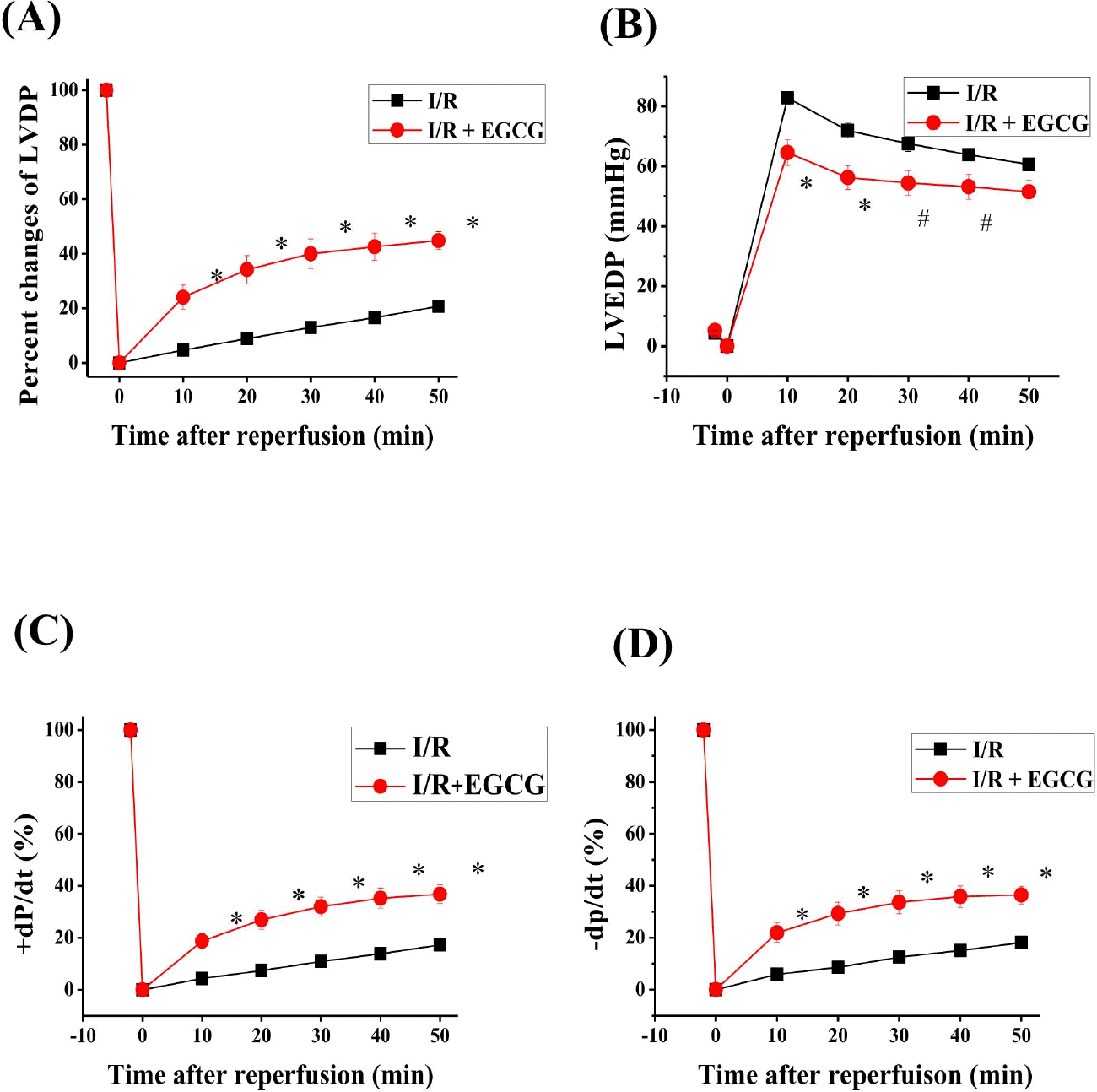 | Fig. 2.Effect of EGCG on LVDP, LVEDP and ±dP/dt in isolated rat heart. EGCG was perfused 10 min before ischemia and during the whole reperfusion period. The percent changes of LVDP (A), LVEDP (B) and ±dP/dt (C, D) during reperfusion. Sham, non-ischemia; I/R, ischemia and reperfusion only; EGCG, ischemia and reperfusion treated with EGCG (5μmol). I/R is square. EGCG is circle. ∗p <0.01 vs. Sham group, #p<0.05 vs. I/R group. |
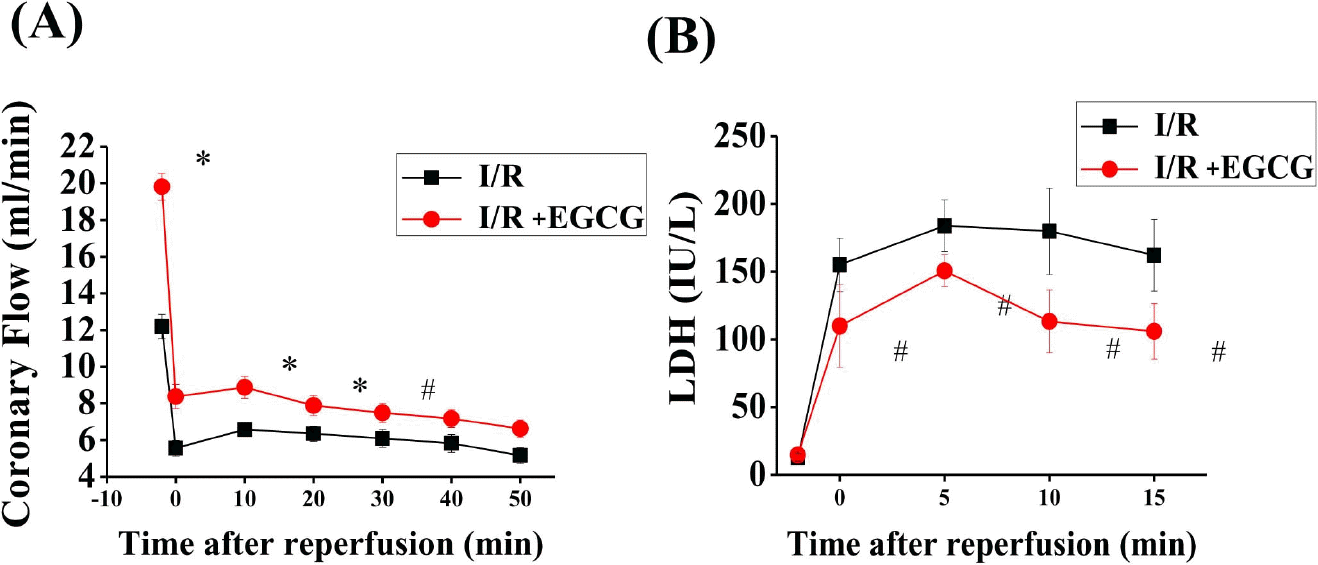 | Fig. 3.Effect of EGCG on coronary flow and LDH in isolated rat hearts. EGCG was perfused 10 min before ischemia and during the whole reperfusion period. The effect of EGCG on coronary flow and LDH before ischemia and during the reperfusion period. Sham, non-ischemia; I/R, ischemia and re-perfusion only; EGCG, ischemia and re-perfusion treated with EGCG (5μmol). ∗p <0.01 vs. Sham group, #p<0.05 vs. I/R group. |
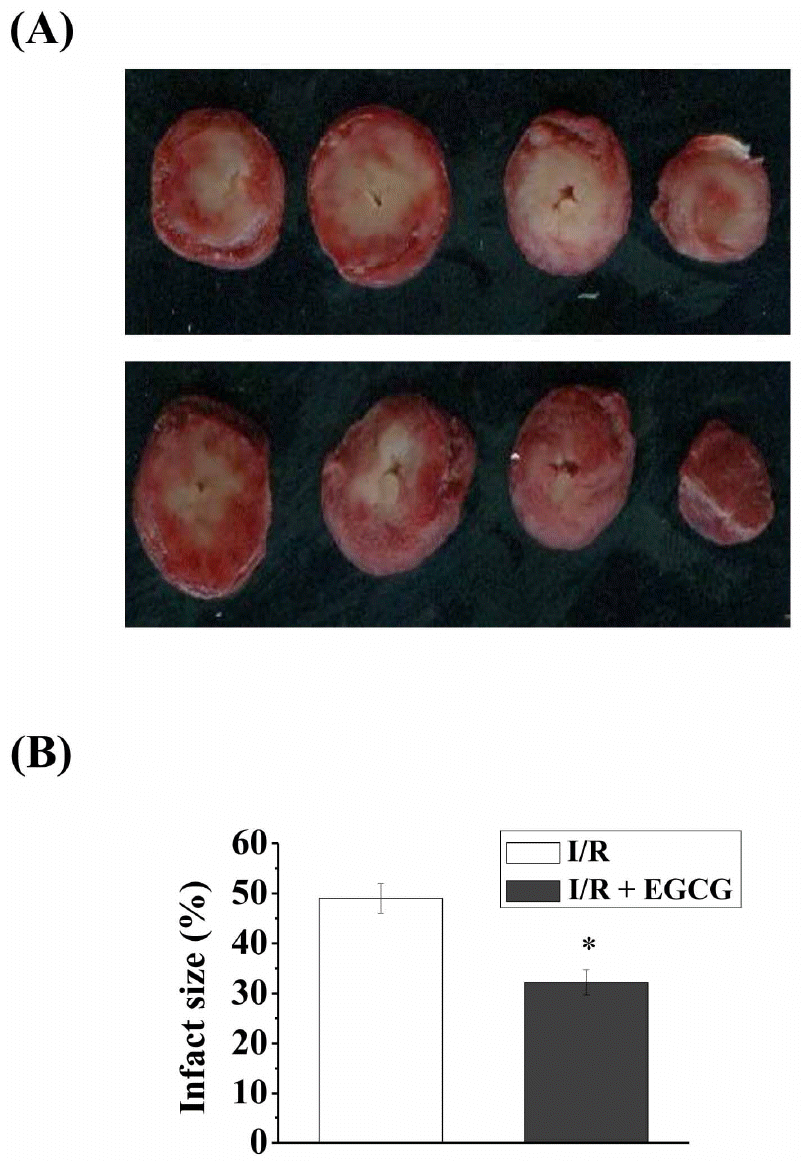 | Fig. 4.Effect of EGCG on infarct size in isolated rat hearts. After 120-min reperfusion, hearts were collected to measure the infarct size by TTC staining (A). Infarct size was expressed as percent of the area-at-risk (AAR). Results were representative of five independent experiments. I/R, ischemia and reperfusion only; EGCG, ischemia and reperfusion treated with EGCG (5μmol). ∗p<0.05 vs. I/R group. |
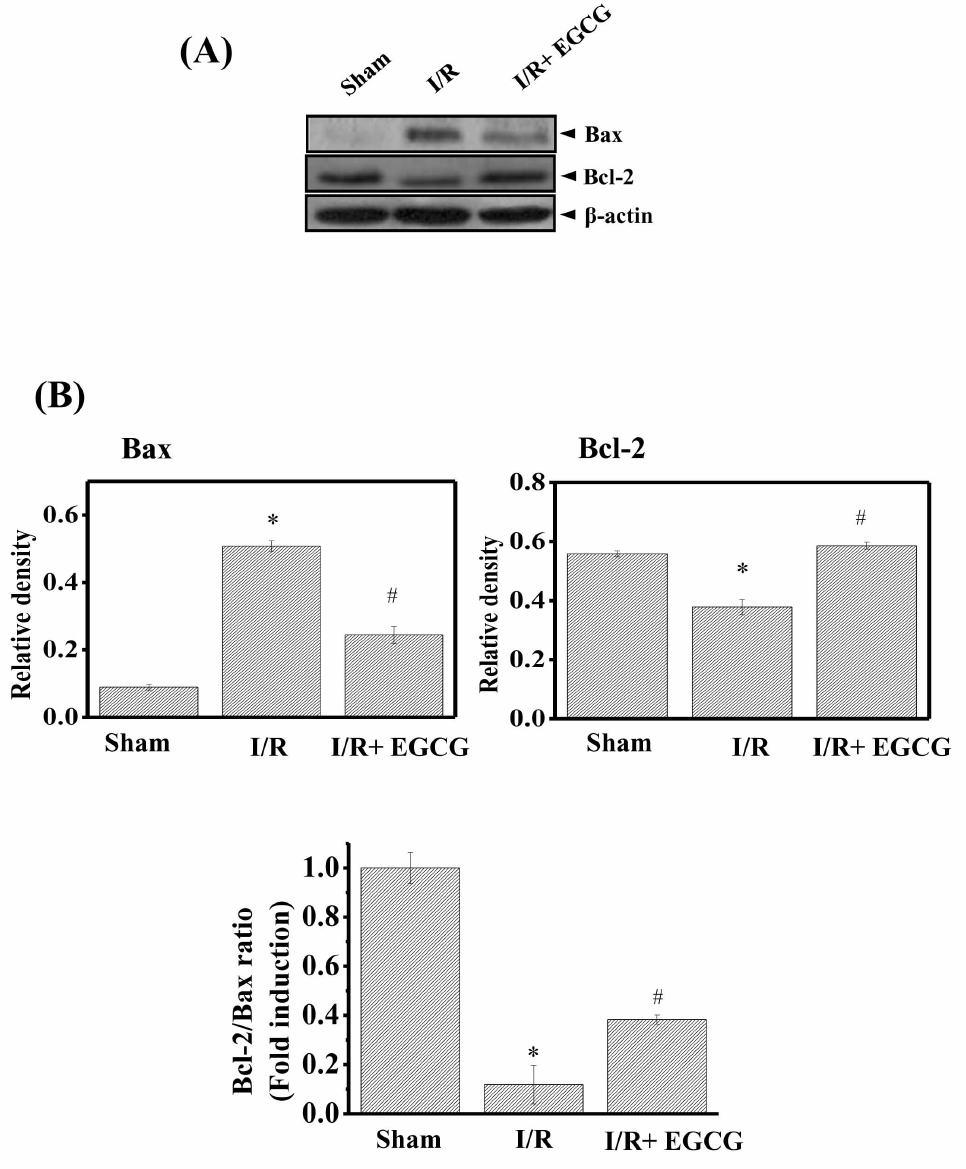 | Fig. 5.Effect of EGCG on Bax and Bcl-2 protein expression levels in isolated rat hearts. After 50-min reperfusion, hearts were collected to measure the protein expression. (A) The expression levels of Bax and Bcl-2 protein were determined by Western blotting. (B) Densitometric analysis of each protein. The ratio of Bcl-2/Bax was shown (lower). Results are representative of three independent experiments. Sham, non-ischemia; I/R, ischemia and reperfusion only; EGCG, ischemia and reperfusion treated with EGCG (5μmol). ∗p<0.05 vs. Sham group, #p<0.05 vs. I/R group. |
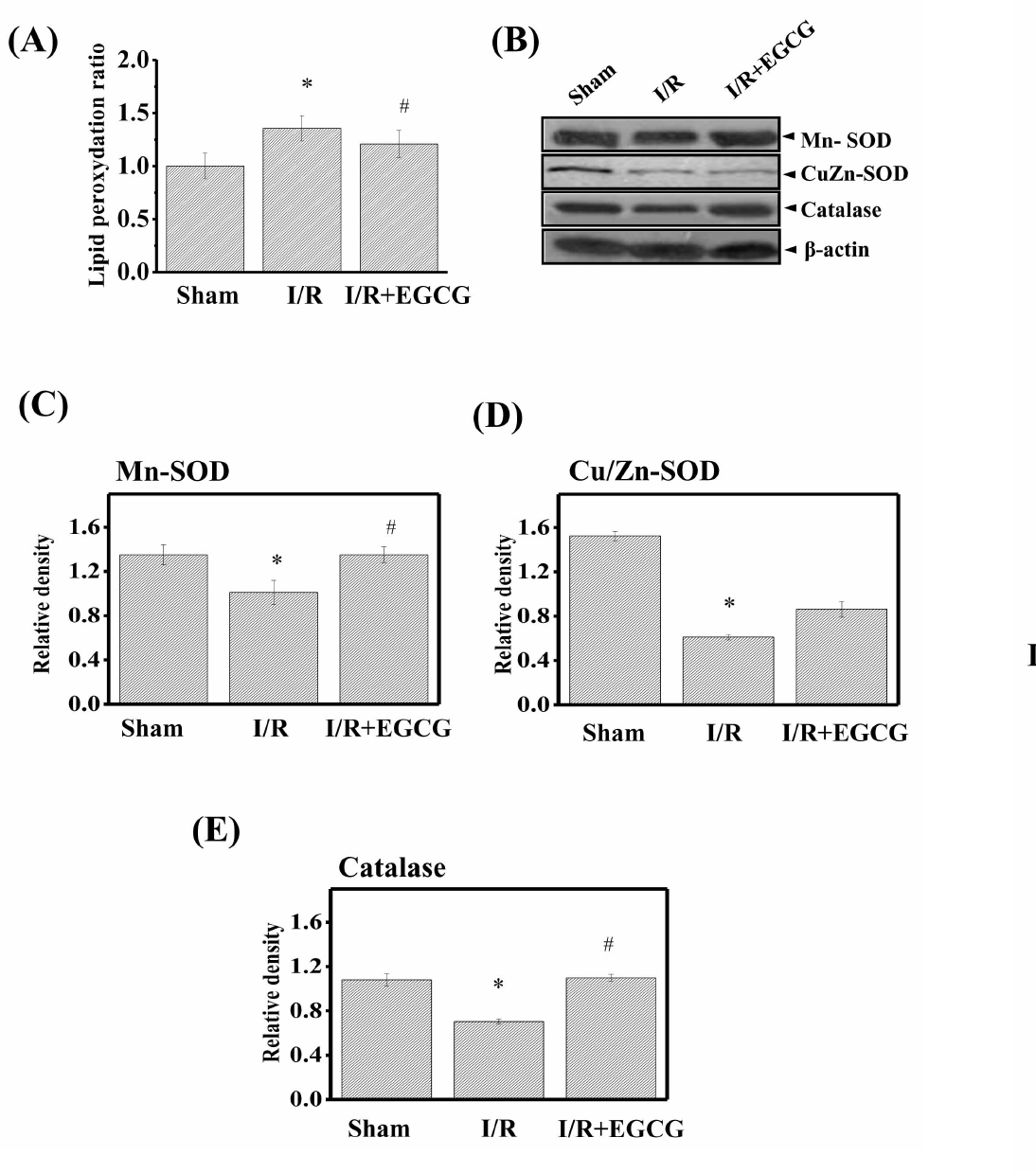 | Fig. 6.Effect of EGCG on lipid peroxydation and Cu/Zn-superoxide dismutase (SOD), Mn-SOD, and catalase protein expression in isolated rat hearts. After 50-min reperfusion, hearts were collected to measure lipid peroxydation (A), the protein expression. (B) The expression levels of Mn-SOD (C), Cu/Zn-SOD (D) and catalase (E) were determined by Western blotting. Results are representative of three independent experiments. Sham, non-ischemia; I/R, ischemia and reperfusion only; EGCG, ischemia and reperfusion treated with EGCG (5μmol). ∗p<0.05 vs. Sham group, #p<0.05 vs. I/R group. |
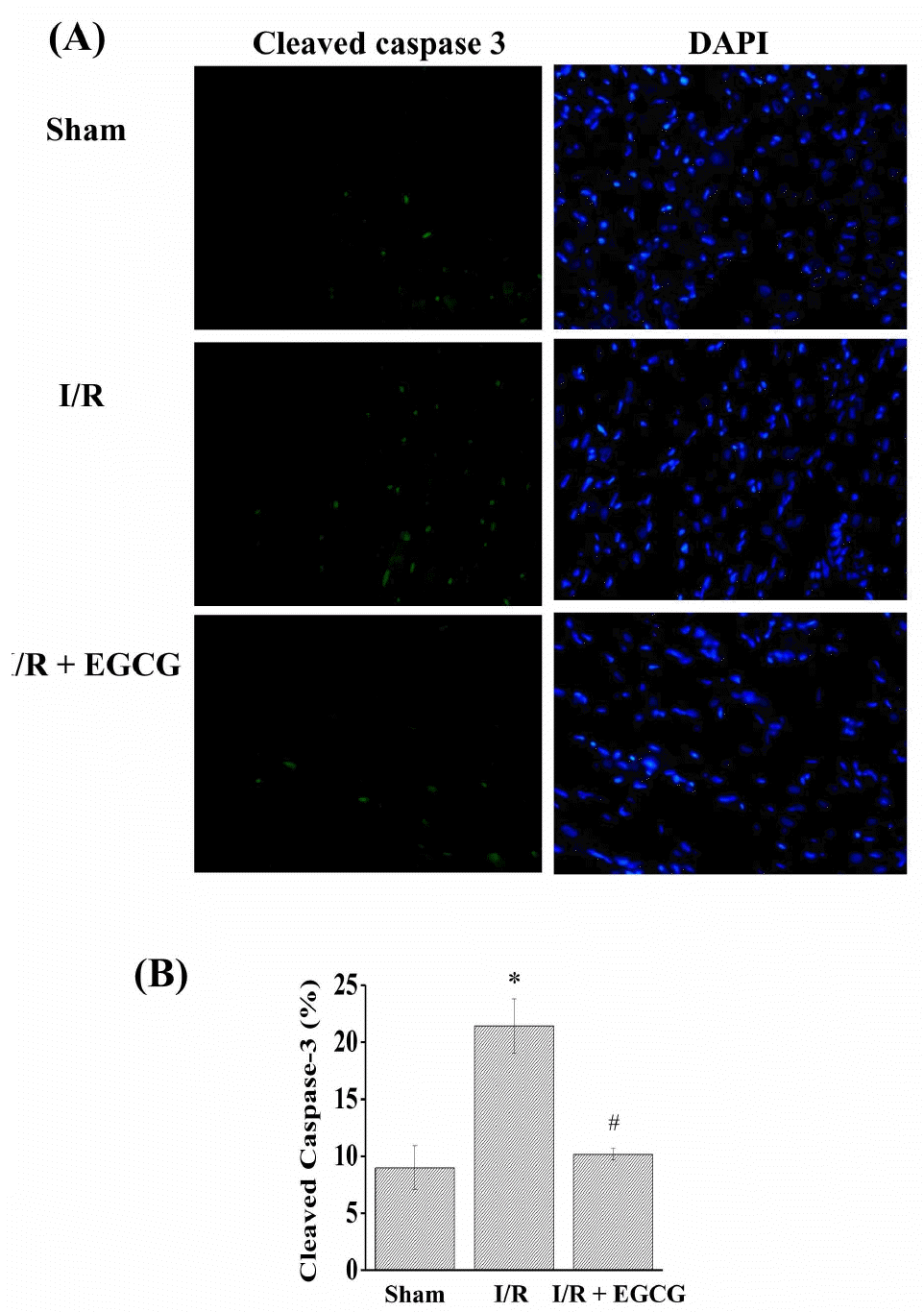 | Fig. 7.Effect of EGCG on caspase-3. Cleaved caspase-3 was measured by immunohistochemical staining methods (A). The ratio of cleaved caspase-3 to DAPI staining (B). Results are representative of three independent experiments. Sham, non-ischemia; I/R, ischemia and reperfusion only; EGCG, ischemia and re-perfusion treated with EGCG (5μmol). ∗p<0.05 vs. Sham group, #p<0.05 vs. I/R group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download