Abstract
Amphetamine, a synthetic psychostimulant, is transported by the dopamine transporter (DAT) to the cytosol and increases the exchange of extracellular amphetamine by intracellular dopamine. Recently, we reported that the phosphorylation levels of ezrin-radixin-moesin (ERM) proteins are regulated by psychostimulant drugs in the nucleus accumbens, a brain area important for drug addiction. However, the significance of ERM proteins phosphorylation in response to drugs of abuse has not been fully investigated. In this study, using PC12 cells as an in vitro cell model, we showed that amphetamine increases ERM proteins phosphorylation and protein kinase C (PKC) β inhibitor, but not extracellular signal-regulated kinase (ERK) or phosphatidylinositol 3-kinases (PI3K) inhibitors, abolished this effect. Further, we observed that DAT inhibitor suppressed amphetamine-induced ERM proteins phosphorylation in PC12 cells. These results suggest that PKCβ-induced DAT regulation may be involved in amphetmaine-induced ERM proteins phosphorylation.
Go to : 
References
1. Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008; 75:76–84.

2. Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009; 32:148–154.
3. White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998; 51:141–153.

4. Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003; 479:153–158.

5. Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008; 154:261–274.

6. Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008; 58:192–208.

7. Kantor L, Park YH, Wang KK, Gnegy M. Enhanced amphetamine-mediated dopamine release develops in PC12 cells after repeated amphetamine treatment. Eur J Pharmacol. 2002; 451:27–35.

8. Park YH, Kantor L, Wang KK, Gnegy ME. Repeated, intermittent treatment with amphetamine induces neurite outgrowth in rat pheochromocytoma cells (PC12 cells). Brain Res. 2002; 951:43–52.

9. Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002; 3:586–599.

10. Louvet-Vallée S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000; 92:305–316.

11. Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008; 40:344–349.

12. Kim WY, Shin SR, Kim S, Jeon S, Kim JH. Cocaine regulates ezrin-radixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience. 2009; 163:501–505.

13. Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006; 138:1289–1298.

14. Shi X, McGinty JF. Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. J Neurochem. 2007; 103:706–713.

15. Chen R, Furman CA, Zhang M, Kim MN, Gereau RW 4th, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009; 328:912–920.
17. Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003; 278:22168–22174.

18. Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005; 280:10914–10919.
19. Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003; 23:8480–8488.

20. Carvelli L, Morón JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002; 81:859–869.

21. Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002; 61:436–445.

22. Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004; 20:1647–1654.

23. Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008; 40:344–349.

24. Kim HS, Bae CD, Park J. Glutamate receptor-mediated phosphorylation of ezrin/radixin/moesin proteins is implicated in filopodial protrusion of primary cultured hippocampal neuronal cells. J Neurochem. 2010; 113:1565–1576.

Go to : 
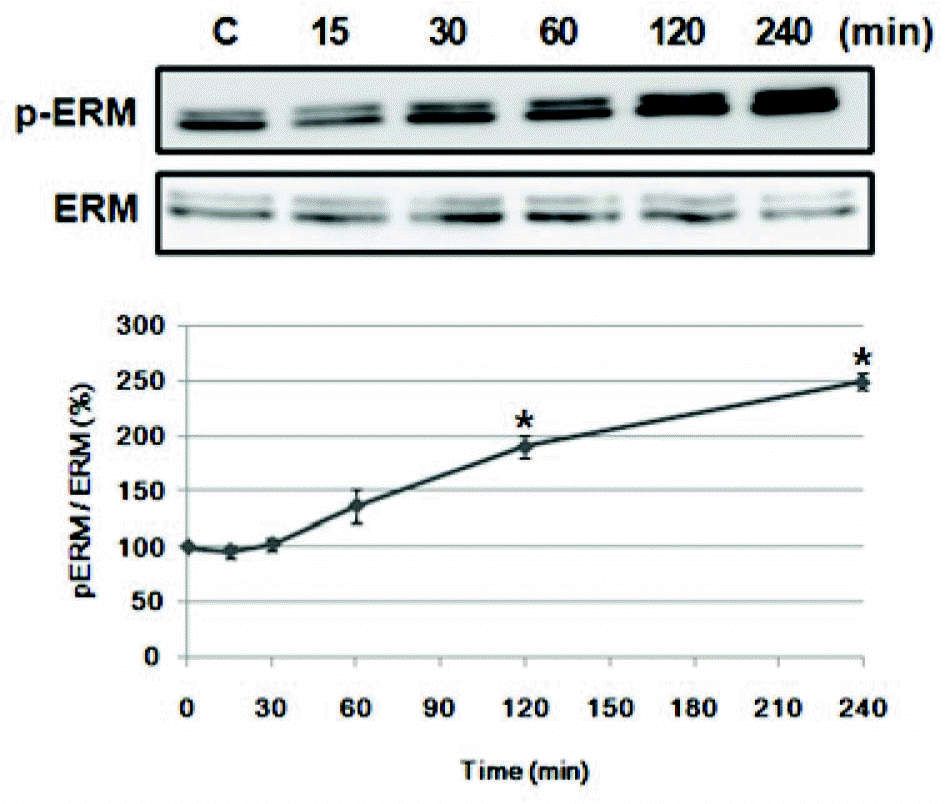 | Fig. 1.Amphetamine induces ERM proteins phosphorylation in PC12 cells. Serum reduced PC12 cells were treated with 5μM of AMPH for the indicated time, and whole cell lysates were immunoblotted with anti-p-ERM or ERM antibody. The intensity of the phosphorylated ERM band was normalized to that of total ERM. The data represent the means±SE of three independent experiments. ∗p<0.05 vs. C. |
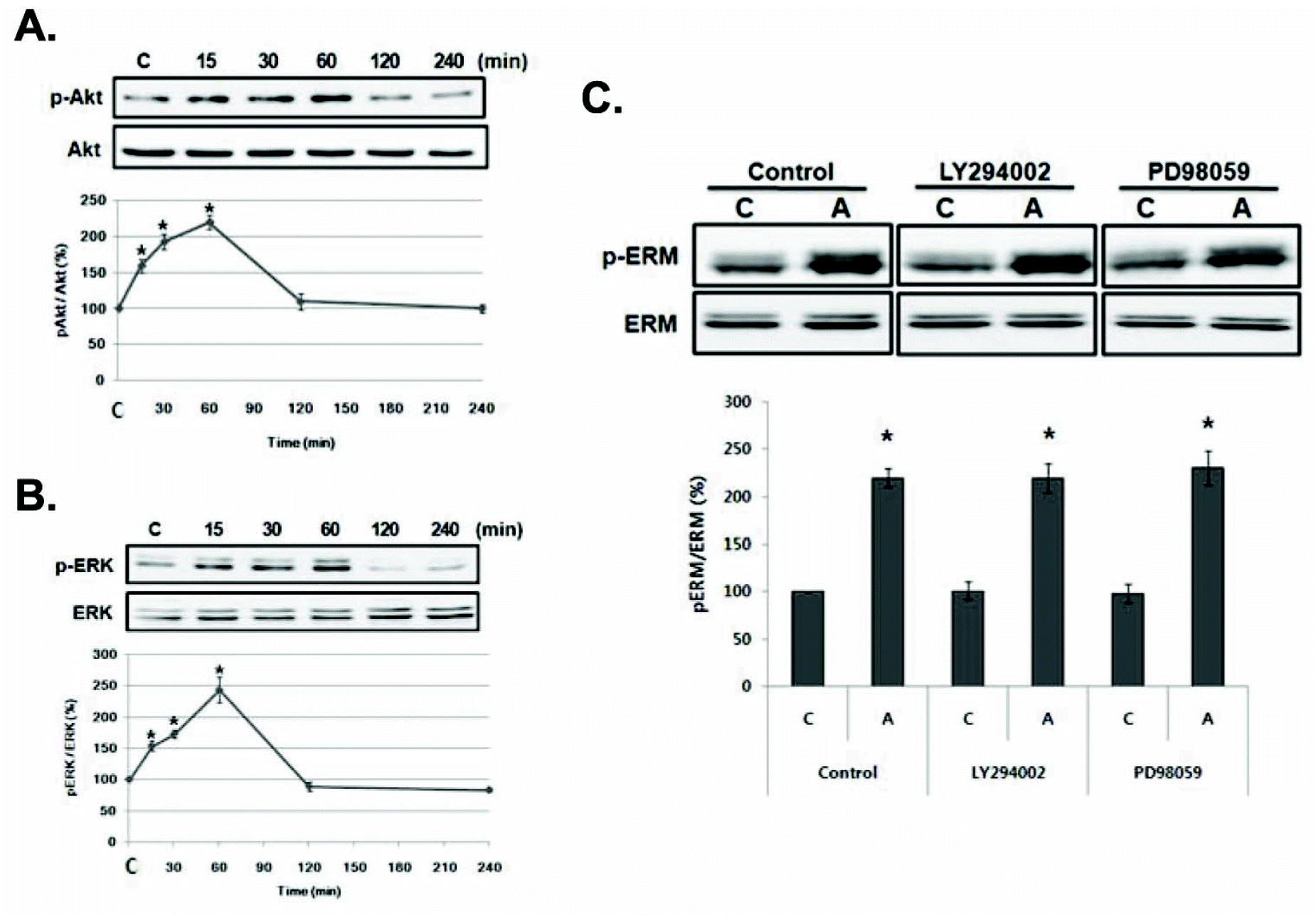 | Fig. 2.Amphetamine-induced ERM proteins phosphorylation is not mediated by Akt or ERK activation in PC12 cells. (A) Serum reduced PC12 cells were treated with 5μM of AMPH for the indicated time, and whole cell lysates were immunoblotted with anti-p-Akt or Akt antibody and (B) anti-p-ERK or ERK antibody. (C) Serum reduced PC12 cells were pre-incubated with 30μM of LY294002 or 10μM of PD098059 for 30 min, and then treated with AMPH for 4 h. The whole cell lysates were immunoblotted with anti-p-ERM or ERM antibody. The intensity of the phosphorylated ERM, Akt and ERK band was normalized to that of total ERM, Akt, and ERK, respectively. The data represent the means±SE of three independent experiments. ∗p<0.05 vs. C.C, unstimulated PC12 cells; A, AMPH-stimulated cells. |
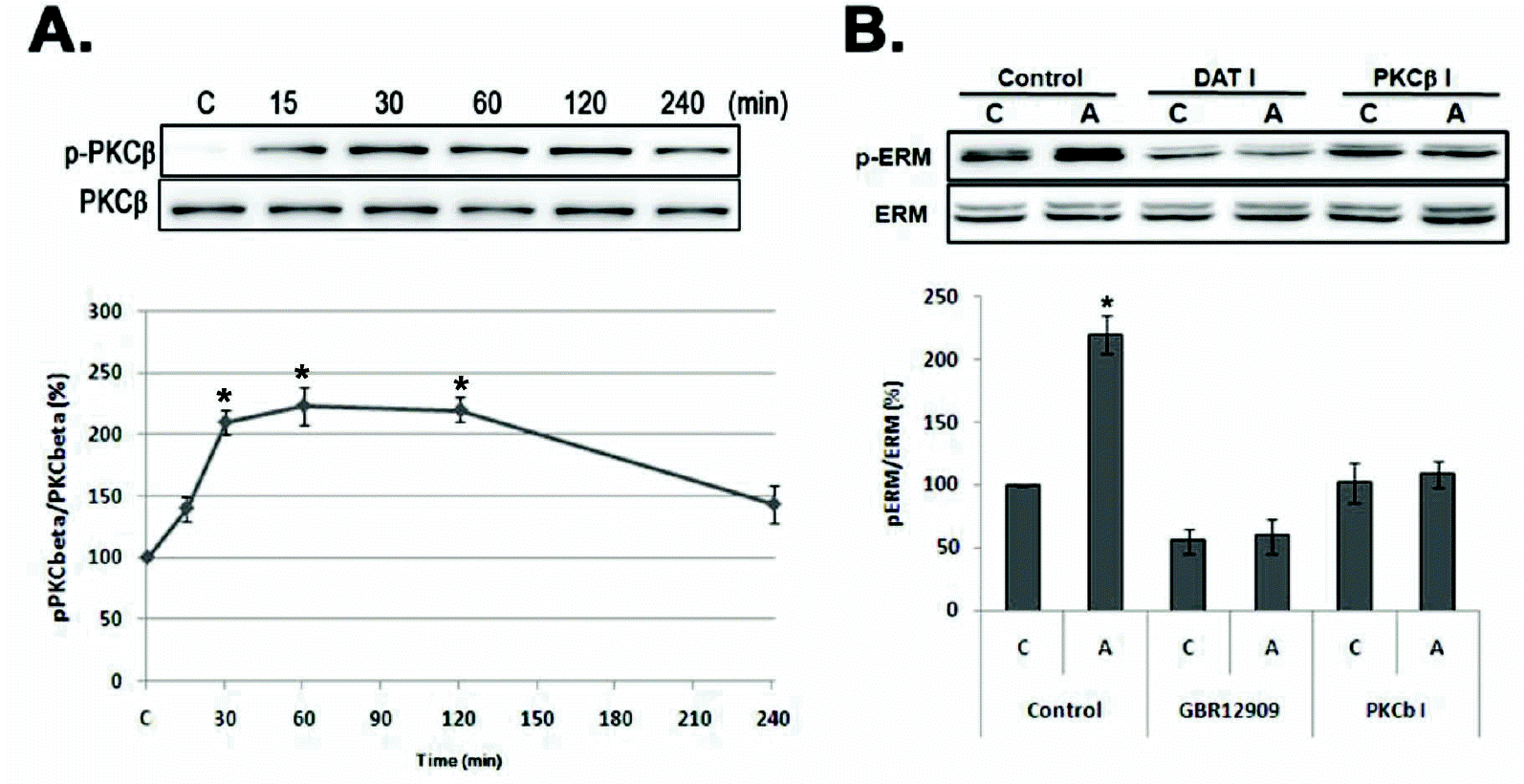 | Fig. 3.Amphetamine-induced ERM proteins phosphorylation is related with PKC β and DAT activation in PC12 cells. (A) Serum reduced PC12 cells were treated with 5 μM of AMPH for the indicated time, and whole cell lysates were immunoblotted with anti-p-PKCβ or PKCβ antibody. (B) PC12 cells were pre-incubated with 10 μM of GBR12909 (DAT I) or 0.5 μM of Enzastaurin (PKCβ I) for 30 min, and then treated with 5 μM of AMPH for 4 h, and whole cell lysates were immunoblotted with indicated antibodies. The intensity of the phosphorylated PKCβ and ERM band was normalized to that of total ERM and PKCβ. The data represent the means±SE of three independent experiments. ∗p<0.05 vs. C, C, unstimulated PC12 cells; A, AMPH-stimulated cells. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download