Abstract
ATP-sensitive K+ channels (KATP) are major component of preventing ischemia-reperfusion injury. However, there is little information regarding to the expressional difference of KATP and its function between left and right ventricles. In this study, we measured the lactate dehydrogenase release of rabbit heart slices in vitro and determined the difference of the KATP expression at the both ventricles by measuring the level of KATP-forming Kir6.2 (OcKir6.2) mRNA using in situ hybridization. The hearts were preconditioned with 15 min hypoxia and reoxygenated for 15 min before a hypoxic period of 60 min, followed by reoxygenation for 180 min. With hypoxic preconditioning (100% N2) with 15 min, left ventricles (LV) showed higher release of LDH comparing with right ventricles (RV). Adding KATP blocker glibenclamide (10μM) prior to a hypoxic period of 60 min, hypoxic preconditioning effect of RV was more abolished than LV. With in situ hybridization, the optical density of OcKir6.2 was higher in RV. Therefore, we suggest that different KATP expression between LV and RV is responsible for the different response to hypoxia and hypoxic preconditioning of rabbit hearts.
Go to : 
References
1. Podesser B, Wollenek G, Seitelberger R, Siegel H, Wolner E, Firbas W, Tschabitscher M. Epicardial branches of the coronary arteries and their distribution in the rabbit heart: the rabbit heart as a model of regional ischemia. Anat Rec. 1997; 247:521–527.

2. Baker JE, Holman P, Gross GJ. Preconditioning in immature rabbit hearts: role of KATP channels. Circulation. 1999; 99:1249–1254.
3. Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998; 97:1848–1867.
4. Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992; 26:1054–1062.

5. Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003; 285:H921–930.

6. Gross GJ. ATP-sensitive potassium channels and myocardial preconditioning. Basic Res Cardiol. 1995; 90:85–88.

7. Seharaseyon J, Sasaki N, Ohler A, Sato T, Fraser H, Johns DC, O'Rourke B, Marbán E. Evidence against functional heteromultimerization of the KATP channel subunits Kir6.1 and Kir6.2. J Biol Chem. 2000; 275:17561–17565.
8. Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002; 99:13278–13283.

9. Park SW, Lee SK, Kim JM, Yoon JS, Kim YH. Effects of quetiapine on the brain-derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neurosci Lett. 2006; 402:25–29.

10. Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K(+) channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002; 283:H1545–1554.
11. Lee KW, Norell MS. Cardiogenic shock complicating myocardial infarction and outcome following percutaneous coronary intervention. Acute Card Care. 2008; 10:131–143.

12. Hochman JS, Buller CE, Sleeper LA, Boland J, Dzavik V, Sanborn TA, Godfrey E, White HD, Lim J, LeJemtel T. Cardiogenic shock complicating acute myocardial infarction-etiologies, management and outcome: a report from the shock trial registry. should we emergently revascularize occluded Coronaries for cardiogenic shock? J Am Coll Cardiol. 2000; 36(3 Suppl A):1063–1070.
13. Brodie BR, Stuckey TD, Hansen C, Bradshaw BH, Downey WE, Pulsipher MW. Comparison of late survival in patients with cardiogenic shock due to right ventricular infarction versus left ventricular pump failure following primary percutaneous coronary intervention for ST-elevation acute myocardial infarction. Am J Cardiol. 2007; 99:431–435.

14. Shuhaiber HJ, Juggi JS, John V, Yousof AM, Braveny P. Differences in the recovery of right and left ventricular function after ischaemic arrest and cardioplegia. Eur J Cardiothorac Surg. 1990; 4:435–440.

15. Mehta SR, Eikelboom JW, Natarajan MK, Diaz R, Yi C, Gibbons RJ, Yusuf S. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001; 37:37–43.
16. Goldstein JA. Right versus left ventricular shock: a tale of two ventricles. J Am Coll Cardiol. 2003; 41:1280–1282.
18. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983; 305:147–148.
19. Grover GJ, Sleph PG, Dzwonczyk S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation. 1992; 86:1310–1316.

Go to : 
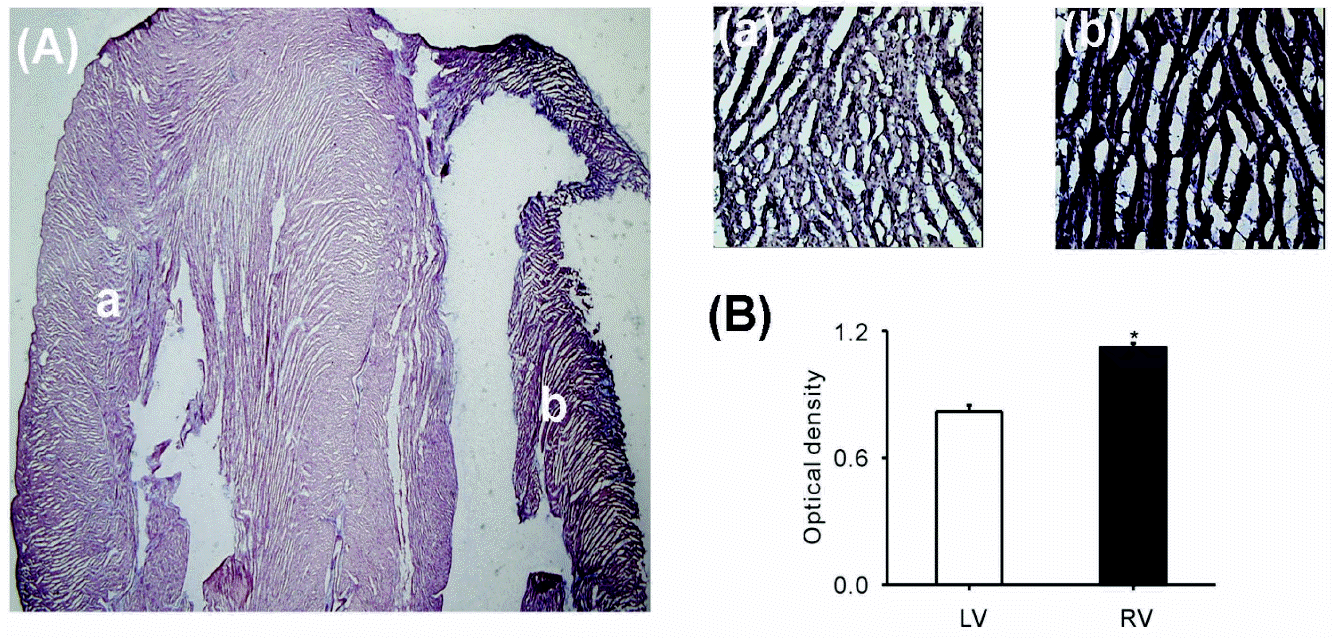 | Fig. 1.mRNA expression of OcKir6.2 in rabbit ventricles. (A) The level of OcKir6.2 expression detected by in situ hybridization was greater in the right ventricle (b) than that in the left ventricle (a). (B) The optical density calculated using ImageJ software was higher in the right than the left ventricle (0.78 vs. 10.5, respectively, n=6, ∗p<0.05). |
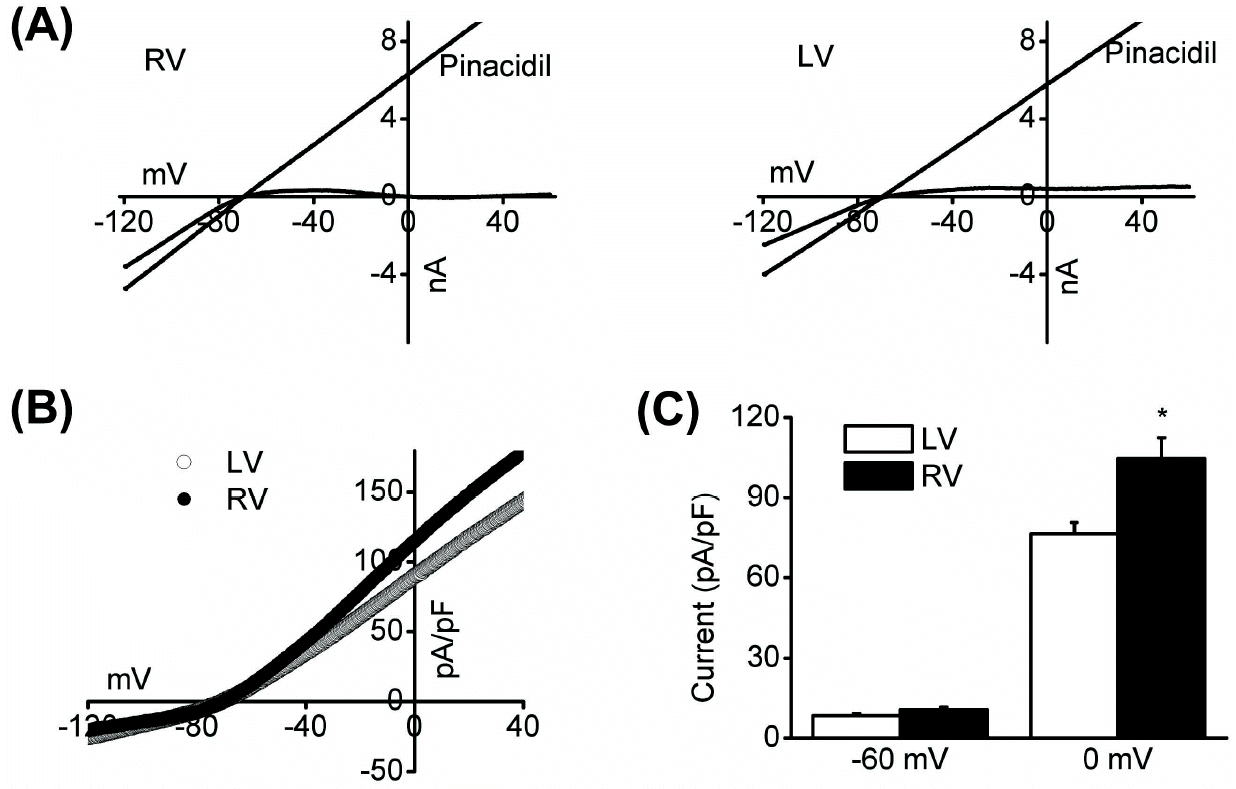 | Fig. 2.Whole-cell KATP channel activity was recorded via ramp pulse (from –120 mV to +60 mV, 0.5 dV/dt) from right and left ventricular myocytes. Representative currents (A) and I-V relationship (B) from right and left myocytes. Application of pinacidil activated KATP channels. (C) Current density at 0 mV was bigger in right than left myocytes (104.7 and 76.3 pA/pF, respectively, n=7, ∗p<0.05). |
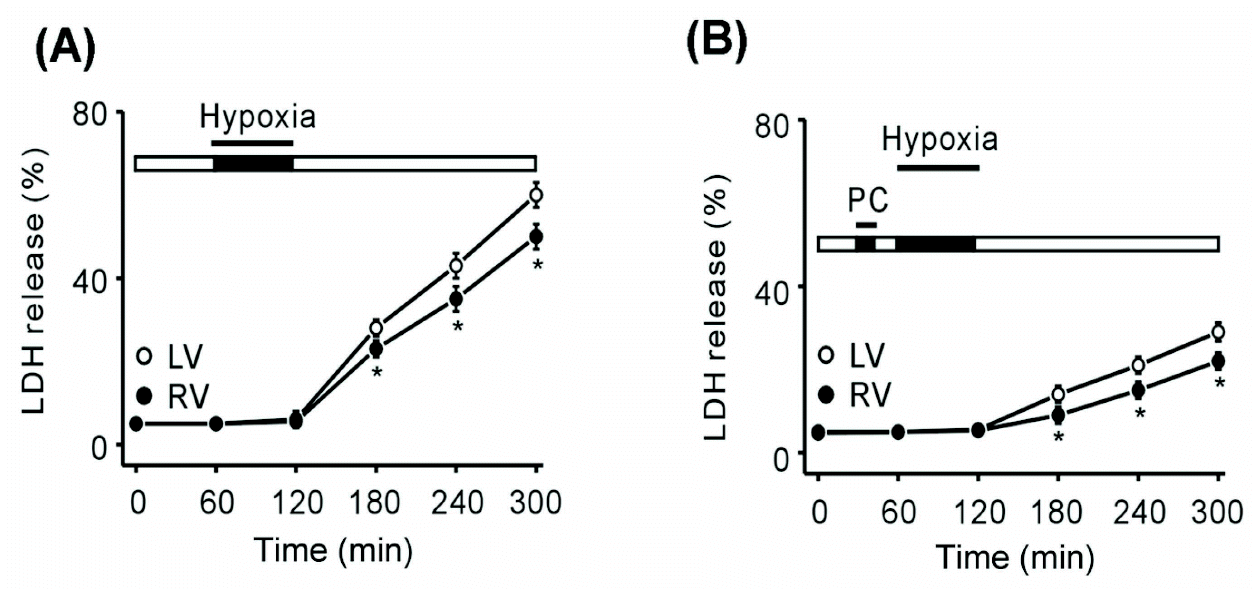 | Fig. 3.LDH release in the left and right ventricles under conditions of hypoxia and hypoxic preconditioning. (A) Measurement of LDH release under hypoxic conditions from 1 to 5 h. LDH release in the left ventricle was higher than that in the right ventricle (58.2% vs. 49.1%, respectively, n=6; ∗p<0.05). (B) Measurement of LDH release in hypoxia after hypoxic preconditioning from 1 to 5 h. Left ventricle LDH release was higher than that in the right ventricle (27.3% and 20%, respectively, n=6; ∗p<0.05). LV (left ventricle, ❍), RV (right ventricle, ), PC, hypoxic preconditioning. |
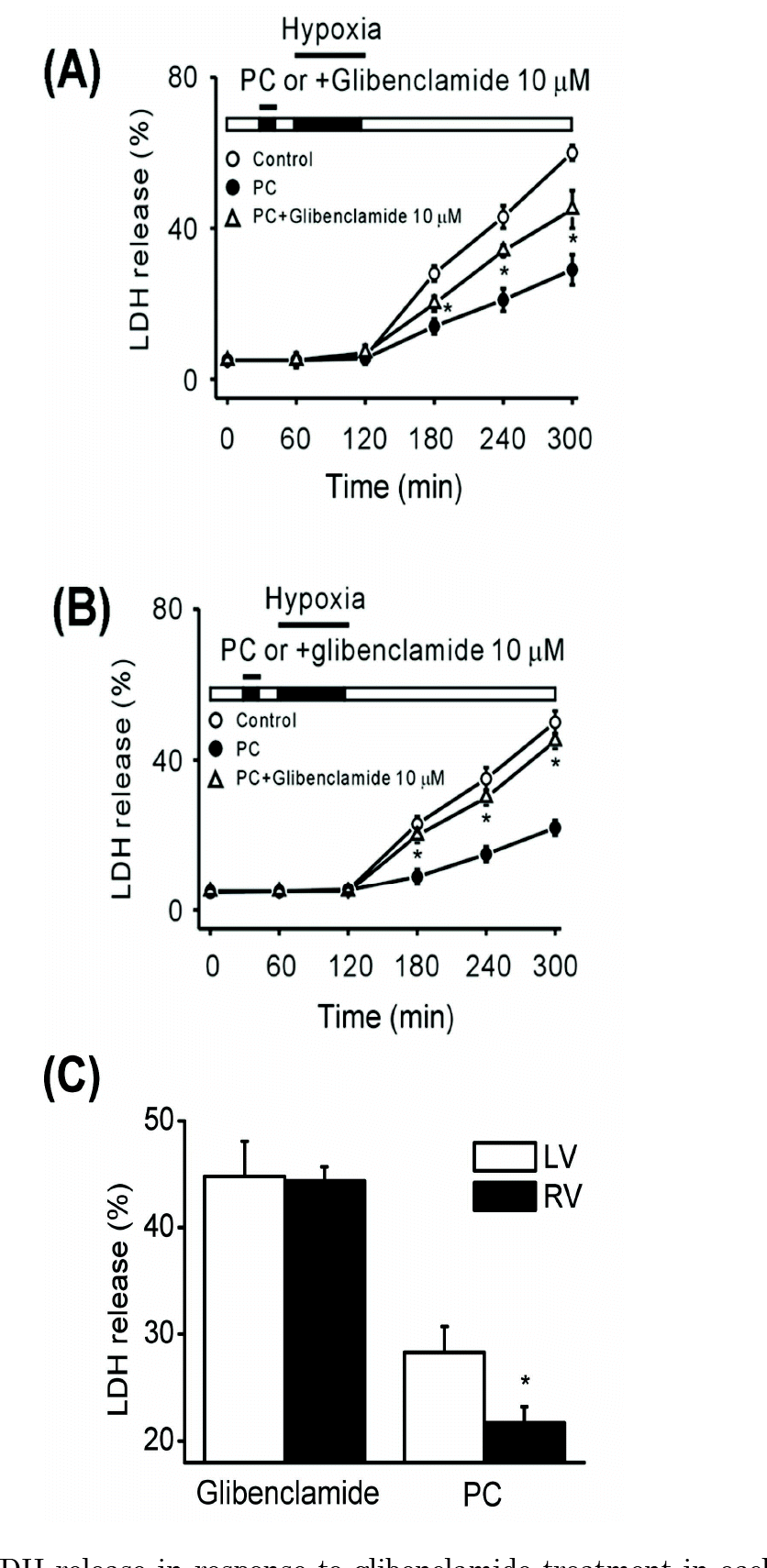 | Fig. 4.LDH release in response to glibenclamide treatment in each ventricle. Differences in LDH release between PC () and PC+ glibenclamide (Δ) were greater in the right ventricle (B) than in the left ventricle (A) (n=6, ∗p<0.05). (C) Glibenclamide sensitivity in the left and right ventricles. LDH release from the left ventricle was greater under PC conditions. However, with addition of glibenclamide, LDH release was not significantly different between the left and right ventricles (n=6, ∗p<0.05). PC, hypoxic preconditioning. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download