Abstract
Extremely low frequency magnetic fields (ELF-MF) have the ability to produce a variety of behavioral and physiological changes in animals. The stomach, as the most sensitive part of the neuroendocrine organ of the gastrointestinal tract, is crucial for the initiation of a full stress response against all harmful stress. Thus, the purpose of this study was to examine whether ELF-MF stimuli induce changes in the activity of neuroendocrine cells, considering their involvement in endocrine or paracrine effect on surrounding cells. The exposure to ELF-MF (durations of 24 h and 1 or 2 weeks, 60 Hz frequency, 0.1 mT intensity) altered the distribution and occurrence of gastrin, ghrelin and somatostatin-positive endocrine cells in the stomach of rats. The change, however, in the secretion of those hormones into blood from endocrine cells did not appear significantly with ELF-MF exposure. Comparing with sham control, ELF-MF exposure for 1 and 2 week induced an increase in BaSO4 suspension propelling ratio of gastrointestinal tract, indicating that ELF-MF affects gastrointestinal motility. Our study revealed that ELF-MF exposure might influence the activity of endocrine cells, an important element of the intrinsic regulatory system in the digestive tract. The pathophysiological character of these changes and the mechanism responsible for neuroendocrine cell are still unclear and require further studies.
Go to : 
References
1. Frey AH. Electromagnetic field interactions with biological systems. FASEB J. 1993; 7:272–281.
3. Janać B, Tovilović G, Tomić M, Prolić Z, Radenović L. Effect of continuous exposure to alternating magnetic field (50 Hz, 0.5 mT) on serotonin and dopamine receptors activity in rat brain. Gen Physiol Biophys. 2009; 28:41–46.
4. Al-Akhras MA. Influence of 50 Hz magnetic field on sex hormones and body, uterine, and ovarian weights of adult female rats. Electromagn Biol Med. 2008; 27:155–163.

5. Sakurai T, Satake A, Sumi S, Inoue K, Miyakoshi J. An extremely low frequency magnetic field attenuates insulin secretion from the insulinoma cell line, RIN-m. Bioelectromagnetics. 2004; 25:160–166.

6. Jelenković A, Janać B, Pesić V, Jovanović DM, Vasiljević I, Prolić Z. Effects of extremely low-frequency magnetic field in the brain of rats. Brain Res Bull. 2006; 68:355–360.

7. Rosen LA, Barber I, Lyle DB. A 0.5 G, 60 Hz magnetic field suppresses melatonin production in pinealocytes. Bioelectromagnetics. 1998; 19:123–127.

8. Yellon SM. Acute 60 Hz magnetic field exposure effects on the melatonin rhythm in the pineal gland and circulation of the adult Djungarian hamster. J Pineal Res. 1994; 16:136–144.

9. Olcese J, Reuss S, Vollrath L. Evidence for the involvement of the visual system in mediating magnetic field effects on pineal melatonin synthesis in the rat. Brain Res. 1985; 333:382–384.

10. Al-Akhras MA, Darmani H, Elbetieha A. Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics. 2006; 27:127–131.

11. De Vita R, Cavallo D, Raganella L, Eleuteri P, Grollino MG, Calugi A. Effects of 50 Hz magnetic fields on mouse spermatogenesis monitored by flow cytometric analysis. Bioelectromagnetics. 1995; 16:330–334.

12. Niehaus M, Brüggemeyer H, Behre HM, Lerchl A. Growth retardation, testicular stimulation, and increased melatonin synthesis by weak magnetic fields (50 Hz) in Djungarian hamsters, Phodopus sungorus. Biochem Biophys Res Commun. 1997; 234:707–711.
13. Nakhilnitskaya ZN, Klimovskaya LD, Kuzmina ZF, Mastryukova VM, Smirnova NP, Strzhizhovsky AD, Cherkasov GV. Possible adaptation to strong magnetic fields. Acta Astronaut. 1983; 10:159–161.

14. Forgács Z, Somosy Z, Kubinyi G, Sinay H, Bakos J, Thuróczy G, Surján A, Hudák A, Olajos F, Lázár P. Effects of whole-body 50-Hz magnetic field exposure on mouse Leydig cells. Scientific World Journal. 2004; 4 Suppl. 2:83–90.

15. Wilson BW, Matt KS, Morris JE, Sasser LB, Miller DL, Anderson LE. Effects of 60 Hz magnetic field exposure on the pineal and hypothalamic-pituitary-gonadal axis in the Siberian hamster (Phodopus sungorus). Bioelectromagnetics. 1999; 20:224–232.
16. Udintsev NA, Serebrov VIu, Tsyrov GI. Effect of an industrial frequency alternating magnetic field on the functional state of the thyroid gland and thyroxine absorption by the organs of rats. Biull Eksp Biol Med. 1978; 86:544–546.
17. Burchard JF, Nguyen DH, Rodriguez M. Plasma concentrations of thyroxine in dairy cows exposed to 60 Hz electric and magnetic fields. Bioelectromagnetics. 2006; 27:553–559.

18. Mostafa RM, Mostafa YM, Ennaceur A. Effects of exposure to extremely low-frequency magnetic field of 2 G intensity on memory and corticosterone level in rats. Physiol Behav. 2002; 76:589–595.
19. Sikiric P, Petek M, Rucman R, Seiwerth S, Grabarevic Z, Rotkvic I, Turkovic B, Jagic V, Mildner B, Duvnjak M, Lang N. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J Physiol Paris. 1993; 87:313–327.
20. Lin H, Head M, Blank M, Han L, Jin M, Goodman R. Myc-mediated transactivation of HSP70 expression following exposure to magnetic fields. J Cell Biochem. 1998; 69:181–188.

21. Lagroye I, Poncy JL. Influences of 50-Hz magnetic fields and ionizing radiation on c-jun and c-fos oncoproteins. Bioelectromagnetics. 1998; 19:112–116.
22. Miyakawa T, Yamada S, Harada S, Ishimori T, Yamamoto H, Hosono R. Exposure of Caenorhabditis elegans to extremely low frequency high magnetic fields induces stress responses. Bioelectromagnetics. 2001; 22:333–339.
23. DiCarlo AL, Farrell JM, Litovitz TA. A simple experiment to study electromagnetic field effects: protection induced by short-term exposures to 60 Hz magnetic fields. Bioelectromagnetics. 1998; 19:498–500.

24. Solcia E, Rindi G, Buffa R, Fiocca R, Capella C. Gastric endocrine cells: types, function and growth. Regul Pept. 2000; 93:31–35.

25. Dockray GJ. The G. W. Harris Prize Lecture. The gut endocrine system and its control. Exp Physiol. 1994; 79:607–634.

26. Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002; 53:39–50.
27. McGuigan JE. Gastric mucosal intracellular localization of gastrin by immunofluorescence. Gastroenterology. 1968; 55:315–327.

28. Schaffer K, McBride EW, Beinborn M, Kopin AS. Interspecies polymorphisms confer constitutive activity to the Mastomys cholecystokinin-B/gastrin receptor. J Biol Chem. 1998; 273:28779–28784.
29. Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004; 18:439–456.

30. Laitl-Kobierska A, Cieślar G, Sieroń A, Grzybek H. Influence of alternating extremely low frequency ELF magnetic field on structure and function of pancreas in rats. Bioelectromagnetics. 2002; 23:49–58.

31. Subbotina TI, Khadartsev AA, Yashin MA, Yashin AA. Effect of rotating electromagnetic fields on proteolytic activity of pepsin in rats. Bull Exp Biol Med. 2004; 137:632–634.

32. Subbotina TI, Khadartsev AA, Yashin MA, Yashin AA. Regulation of proteolytic activity of pepsin in rats [correction of mice] by rotating electromagnetic field. Bull Exp Biol Med. 2005; 139:316–318.
33. Schimmelpfeng J, Dertinger H. Action of a 50 Hz magnetic field on proliferation of cells in culture. Bioelectromagnetics. 1997; 18:177–183.

34. Funk RH, Monsees TK. Effects of electromagnetic fields on cells: physiological and therapeutical approaches and molecular mechanisms of interaction. A review. Cells Tissues Organs. 2006; 182:59–78.
35. Tzaneva MA. Ultrastructural immunohistochemical localization of gastrin, somatostatin and serotonin in endocrine cells of human antral gastric mucosa. Acta Histochem. 2003; 105:191–201.

Go to : 
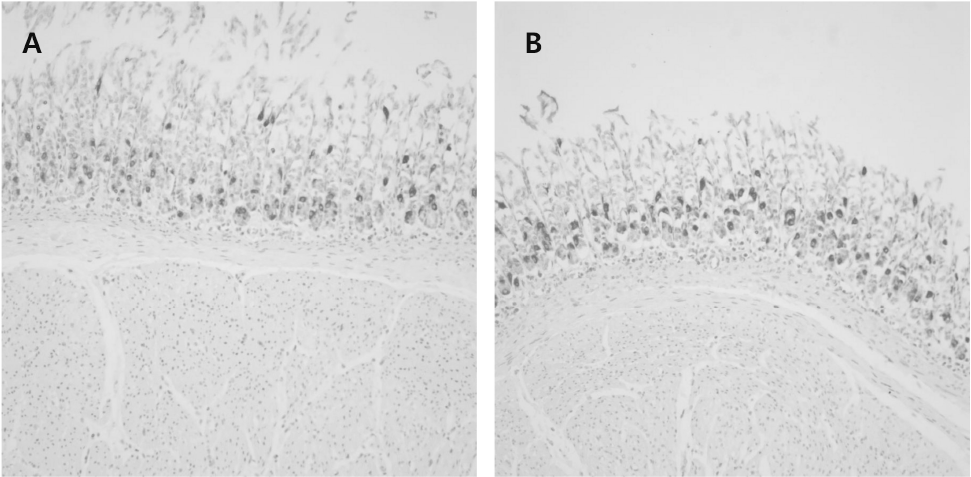 | Fig. 1.Gastrin-positive cells mainly in the basal portion of the antral mucosa. (A) Sham control, (B) ELF-MF (durations of 24 h and 2 weeks, 60 Hz frequency, 0.1 mT intensity) exposure, ×200. |
 | Fig. 2.Ghrelin-positive cells mainly in the basal portion of the antral mucosa. (A) Sham control, (B) ELF-MF (durations of 24 h and 2 weeks, 60 Hz frequency, 0.1 mT intensity) exposure, ×200. |
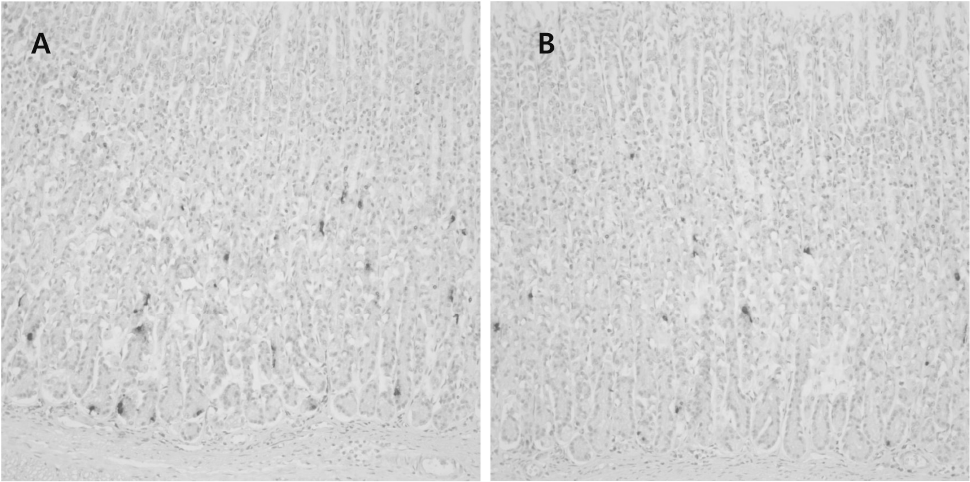 | Fig. 3.Somatostatin-positive cells mainly in the basal portion of the antral mucosa. (A) Sham control, (B) ELF-MF (durations of 24 h and 2 weeks, 60 Hz frequency, 0.1 mT intensity) exposure, ×200. |
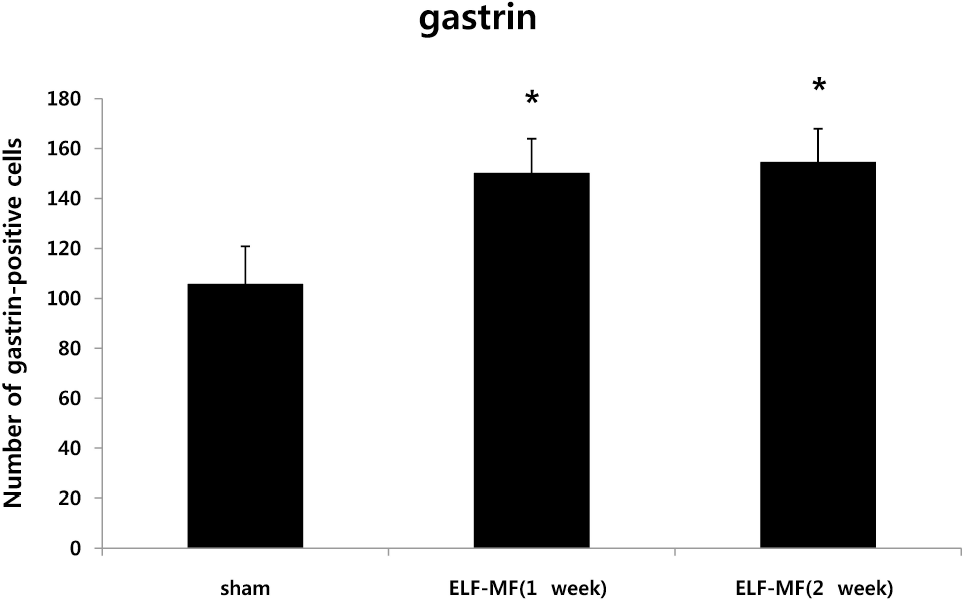 | Fig. 4.Changes in the number of gastrin-positive neuroendocrine cells in rat stomach. Data represent mean±SD, n=7. ∗p<.05 versus sham. |
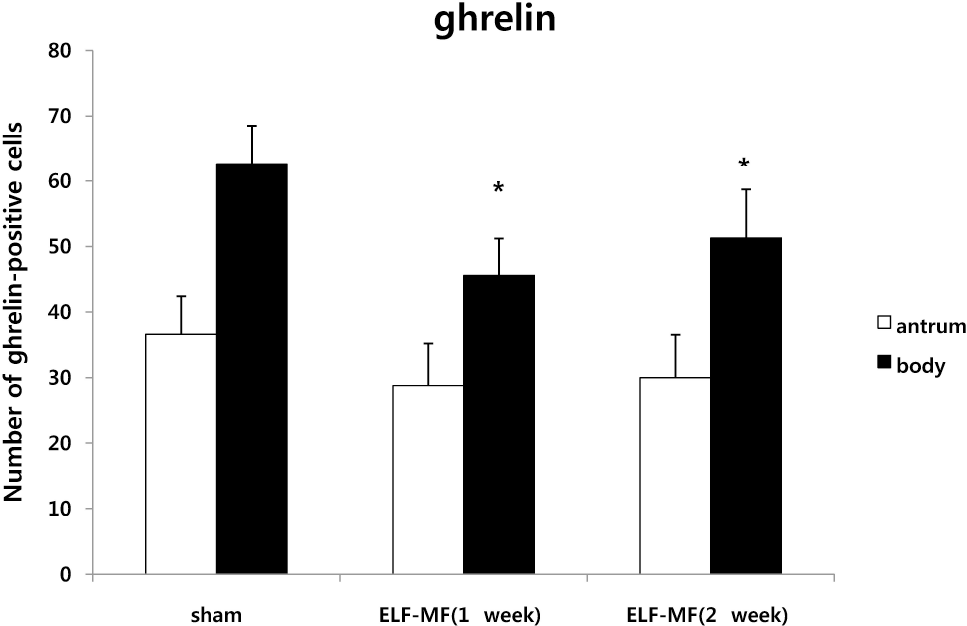 | Fig. 5.Changes in the number of ghrelin-positive neuroendocrine cells in rat stomach. Data represent mean±SD, n=7. ∗p<.05 versus sham. |
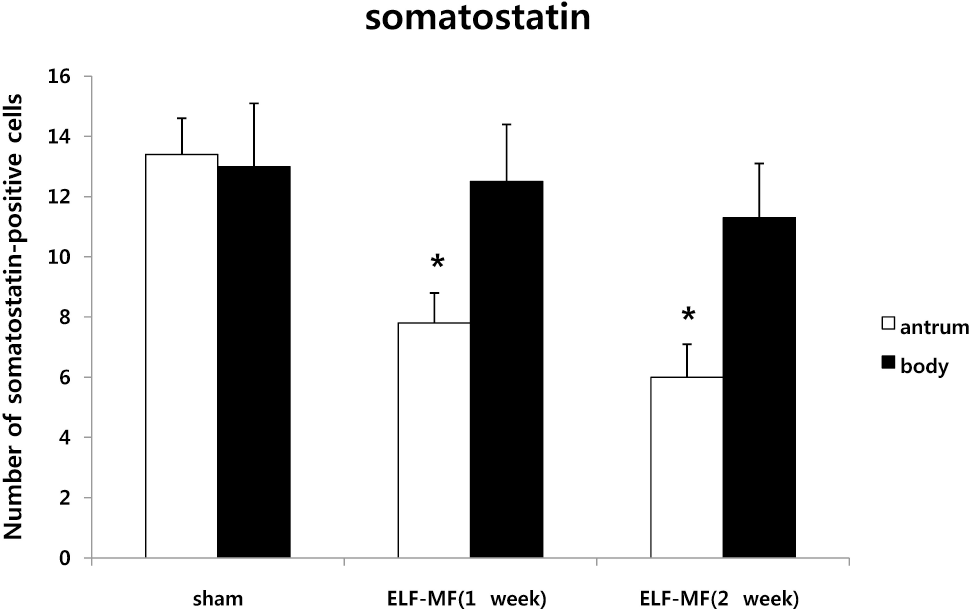 | Fig. 6.Changes in the number of somatostatin-positive neuro-endocrine cells in rat stomach. Data represent mean±SD, n=7. ∗p<.05 versus sham. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download