Abstract
We demonstrate that glycoprotein isolated from Dioscorea batatas (GDB) has immunostimulatory effects including macrophage activation. Analysis of infiltration of inflammatory cells into peritoneal cavity showed GDB treatment significantly increased the recruitment of macrophages, lymphocytes, neutrophils, and monocytes into the peritoneal cavity. Treatment of spleen cells isolated from C57BL/6 mice with GDB significantly increased the proliferation of B cells and T cells induced by LPS and ConA, respectively. Treatment with GDB significantly increased the cytolytic capacity of NK cells and macrophages against YAC-1 and B16 cells, respectively. In order to further confirm and investigate the mechanism of GDB on macrophage activation, we analyzed the effects of GDB on the cytokine expression including iNOS, IL-1β, and TNF-α in mouse macrophage cell line, RAW 264.7 cells. RT-PCR and ELISA showed that GDB increased the expression of IL-1β, and TNF-α, whereas iNOS was not induced by GDB. Collectively, this series of experiments indicates that GDB stimulates immune system including macrophage activation.
Go to : 
References
1. Jin UH, Kim DI, Lee TK, Lee DN, Kim JK, Lee IS, Kim CH. Herbal formulation, Yukmi-jihang-tang-Jahage, regulates bone resorption by inhibition of phosphorylation mediated by tyrosine kinase Src and cyclooxygenase expression. J Ethnopharmacol. 2006; 106:333–343.

2. Chen H, Wang C, Chang CT, Wang T. Effects of Taiwanese yam (Dioscorea japonica Thunb var. pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition. 2003; 19:646–651.

3. Higuchi M, Higashi N, Taki H, Osawa T. Cytolytic mechanisms of activated macrophages. Tumor necrosis factor and L-arginine-dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J Immunol. 1990; 144:1425–1431.
4. Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989; 169:1543–1555.

5. Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am J Pharm Educ. 2006; 70:102.

6. Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993; 90:9730–9734.

7. Jeon YJ, Kim YK, Lee M, Park SM, Han SB, Kim HM. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-kappaB/Rel in lipopoly-saccharide-stimulated macrophages. J Pharmacol Exp Ther. 2000; 294:548–554.
8. Lee KY, Jeon YJ. Polysaccharide isolated from Poria cocos sclerotium induces NF-kappaB/Rel activation and iNOS expression in murine macrophages. Int Immunopharmacol. 2003; 3:1353–1362.
9. Li MH, Kothandan G, Cho SJ, Huong PT, Nan YH, Lee KY, Shin SY, Yea SS, Jeon YJ. Magnolol Inhibits LPS-induced NF-κ B/Rel Activation by Blocking p38 Kinase in Murine Macrophages. Korean J Physiol Pharmacol. 2010; 14:353–358.
10. Gaidamashvili M, Ohizumi Y, Iijima S, Takayama T, Ogawa T, Muramoto K. Characterization of the yam tuber storage proteins from Dioscorea batatas exhibiting unique lectin activities. J Biol Chem. 2004; 279:26028–26035.

11. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

12. Klimetzek V, Remold HG. The murine bone marrow macrophage, a sensitive indicator cell for murine migration inhibitory factor and a new method for their harvest. Cell Immunol. 1980; 53:257–266.

13. Gifford GE, Flick DA, AbdAllah NA, Fisch H. Production of a cytotoxin from phorbol myristate acetate-treated human promyelocytes. J Natl Cancer Inst. 1984; 73:69–73.
14. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138.

15. Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Statist Assoc. 1955; 50:1096–1121.

16. Hibbs JB Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987; 235:473–476.

17. Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984; 2:283–318.

18. Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, Le Beau MM. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988; 239:497–500.

19. Thornton BP, Větvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol. 1996; 156:1235–1246.
20. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69:11–25.

21. Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999; 11:13–18.

22. Lin PL, Lin KW, Weng CF, Lin KC. Yam storage protein dioscorins from dioscorea alata and dioscorea japonica exhibit distinct immunomodulatory activities in mice. J Agric Food Chem. 2009; 57:4606–4613.

23. Fu SL, Hsu YH, Lee PY, Hou WC, Hung LC, Lin CH, Chen CM, Huang YJ. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem Biophys Res Commun. 2006; 339:137–144.

25. Oh PS, Lim KT. HeLa cells treated with phytoglycoprotein (150 kDa) were killed by activation of caspase 3 via inhibitory activities of NF-kappaB and AP-1. J Biomed Sci. 2007; 14:223–232.
26. Lee SJ, Lim KT. Phytoglycoprotein inhibits interleukin-1beta and interleukin-6 via p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated RAW 264.7 cells. Naunyn Schmiedebergs Arch Pharmacol. 2008; 377:45–54.
Go to : 
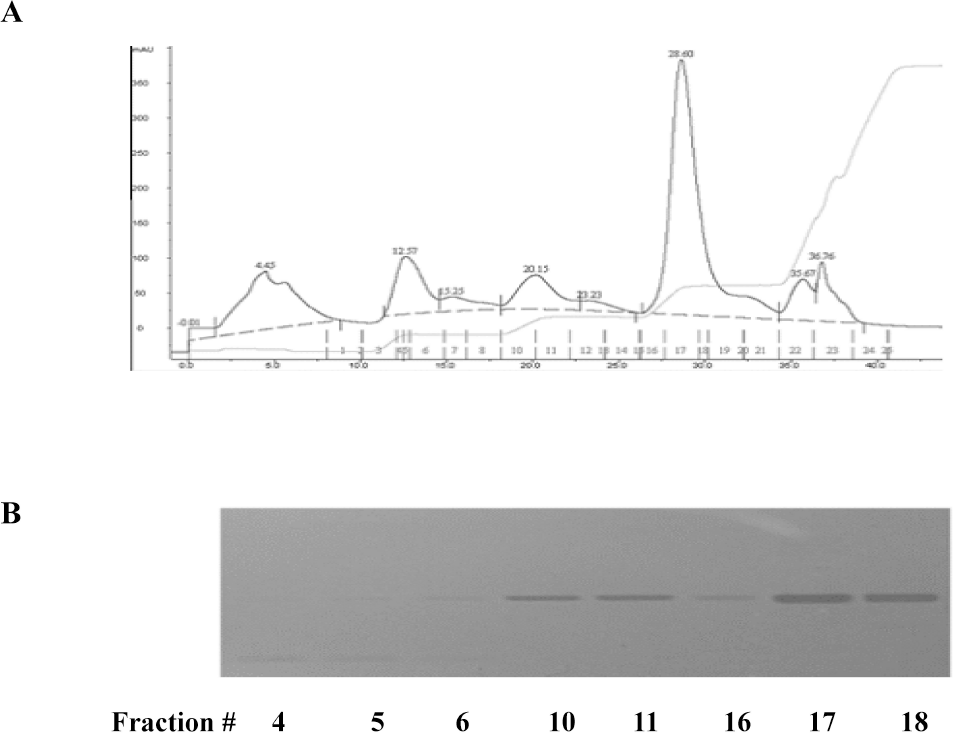 | Fig. 1.Characterization of glycoprotein isolated from Dioscorea batatas. (A) The lyophilized proteins were dissolved in 50 mM Tris-HCl buffer (pH 8.0) and subjected to an anion-exchange chromatography. Fractionation of proteins was performed by a step-wise salt gradient (0.1, 0.2, 0.3, and 1 M) NaCl in equilibrium buffer. (B) Peak fractions were further analyzed by SDS-PAGE. |
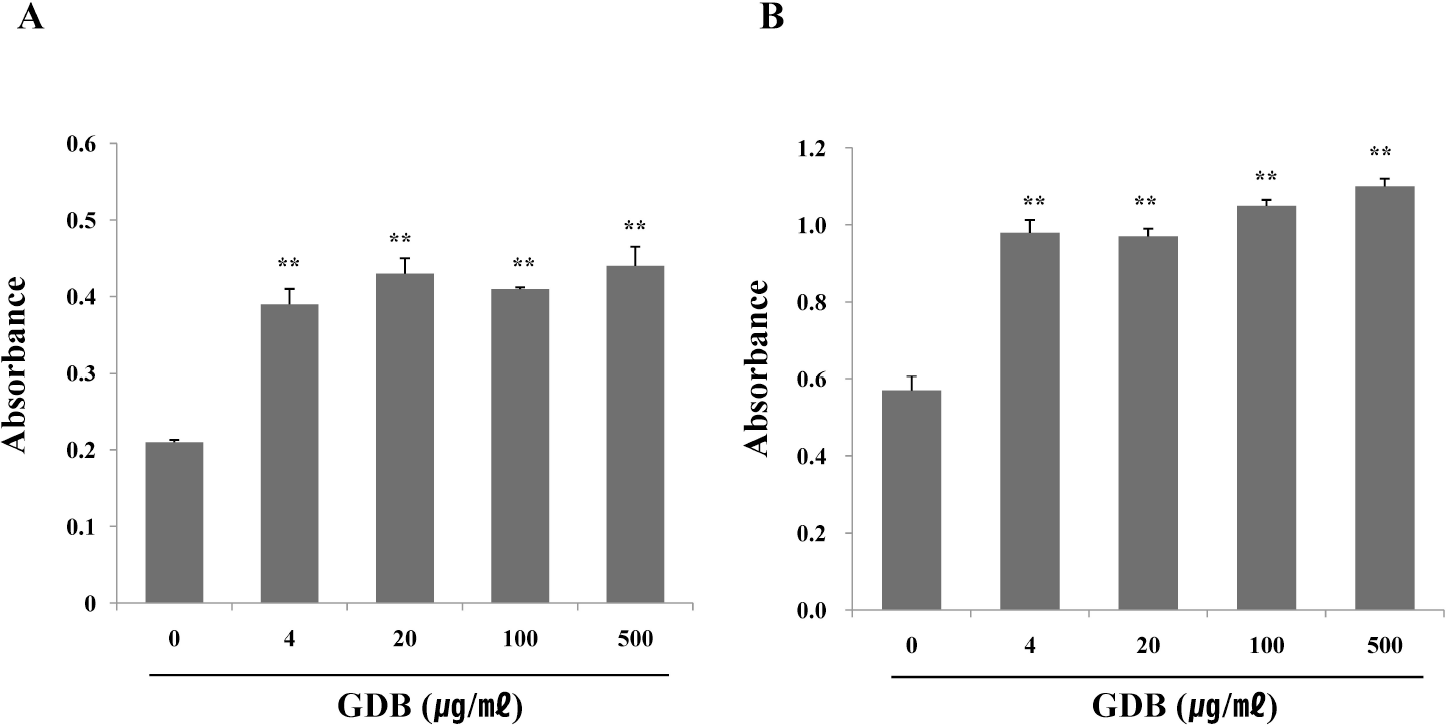 | Fig. 2.Effect of GDB on (A) LPS- and (B) ConA-stimulated splenic lymphocyte proliferation. Splenocytes were isolated from C57BL/6 mice. The results are presented as the mean±S.E.M. ∗∗Denote significant differences (p<0.05, p<0.01) vs. the control group. Experiments were repeated three times. |
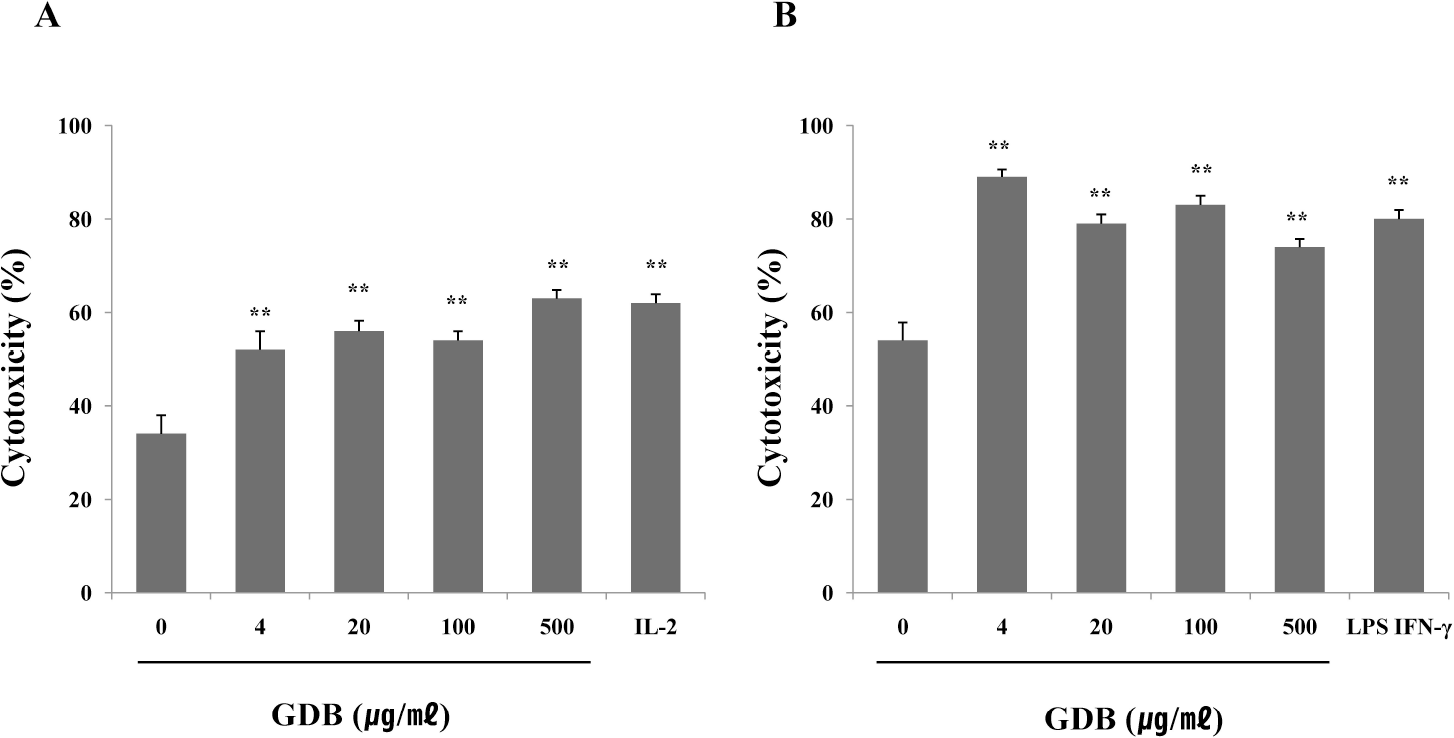 | Fig. 3.Effects of GDB on (A) NK cell- and (B) macrophage-mediated cytotoxicity. Cytotoxicity was measured as described in Methods and was expressed as the cytolytic percentage of target cells. IL-2 and LPS/IFN-γ were used as a positive control of NK cell- and macrophage-mediated cytotoxicity, respectively. The results are presented as the mean±S.E.M. ∗∗Denotes significant differences (p <0.01) vs. the control group. Experiments were repeated three times. |
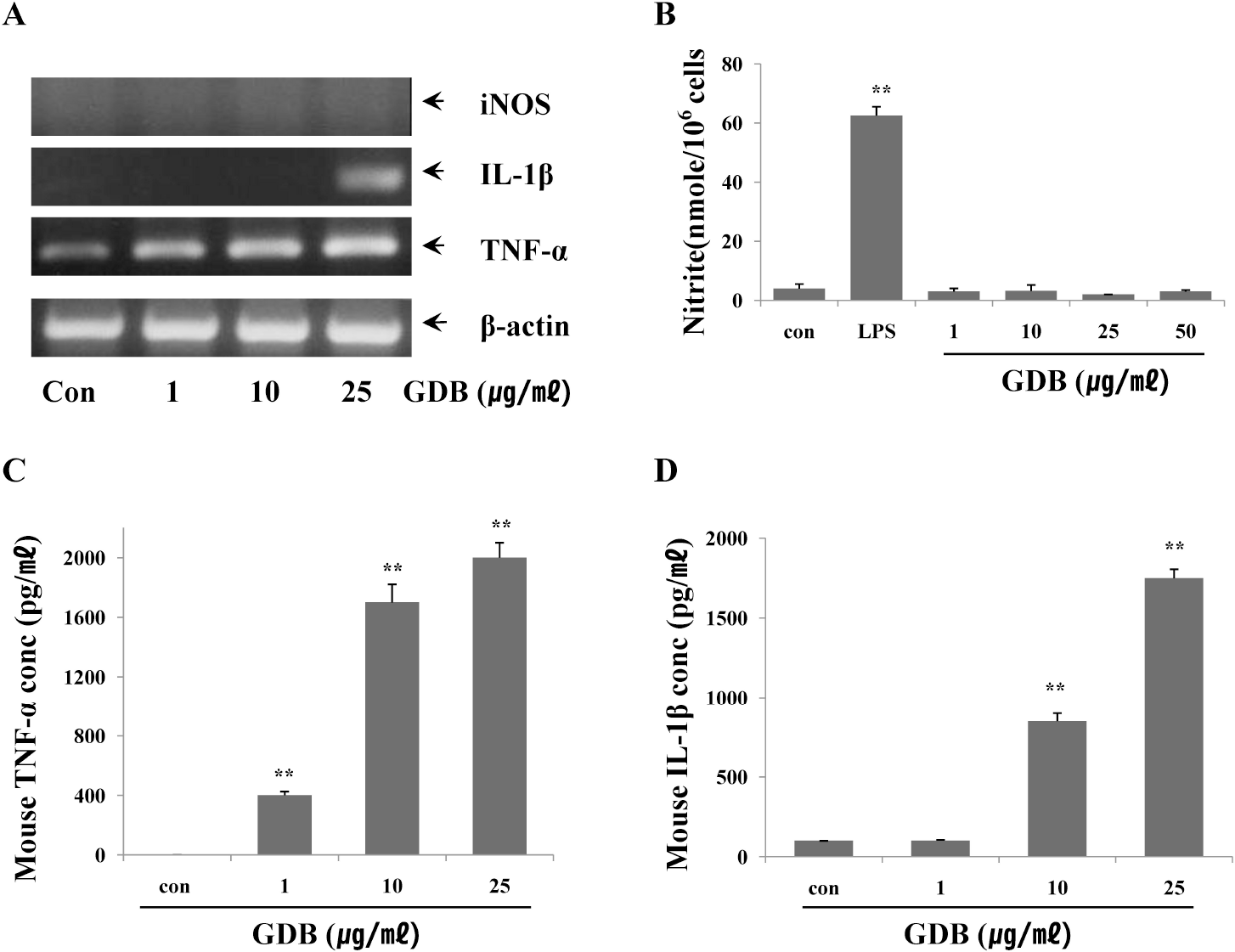 | Fig. 4.Effects of GDB on macrophage activation. (A) RAW264.7 cells were treated with GDB for 8 h. Total RNA was then isolated and analyzed for iNOS, IL-0, and TNF-α using RT-PCR. The production of (B) NO2–, (C) TNF-α, and (D) IL-1β were analyzed from the cell lysates treated with GDB for 24 h. The results are presented as the mean± S.E.M. ∗∗Denotes significant differences (p<0.01) vs. the control group. One representative of three experiments is shown. |
Table 1.
Effect of GDB on the recruitment of inflammatory cells
| Control | Saline | 25 mg/kg | 50 mg/kg | 100 mg/kg | 200 mg/kg | |
|---|---|---|---|---|---|---|
| Macrophage (×106) | 0.27±0.01 | 0.84±0.04∗∗ | 1.00±0.12∗ | 1.07±0.02∗∗,†† | 1.20±0.11∗∗,† | 1.01±0.09∗ |
| Lymphocyte (×106) | 0.59±0.07 | 1.00±0.07∗ | 1.67±0.09∗∗++ | 1.67±0.08∗∗†† | 1.89±0 1.5∗∗,†† | 1.70±0.1 ∗∗†† |
| Neutrophil (×106) | 0.26±0.02 | 0.82±0.08∗∗ | 1.24±0.09∗∗ + | 0.99±0.13∗∗ | 1.26±0.1∗∗,† | 1.40±0.26∗∗† |
| Eosinophil (×106) | 0.01±0.01 | 0.28±0.03∗∗ | 0.28±0.04∗ | 0.42±0.08 | 0.37±0.03∗ | 0.43±0.07∗∗ |
| Monocyte (×106) | 0.23±0.04 | 0.42±0.04∗ | 0.78±0.01∗∗, + | 0.93±0.02∗∗†† | 1.01±0.2∗ | 1 1 4±0 07∗∗, †† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download