Abstract
In this study, we studied whether hydrogen sulfide (H2S) has an effect on the pacemaker activity of interstitial cells of Cajal (ICC), in the small intestine of mice. The actions of H2S on pacemaker activity were investigated using whole-cell patch-clamp technique, intracellular Ca2+ analysis at 30°C and RT-PCR in cultured mouse intestinal ICC. Exogenously applied sodium hydrogen sulfide (NaHS), a donor of hydrogen sulfide, caused a slight tonic inward current on pacemaker activity in ICC at low concentrations (50 and 100 μM), but at high concentration (500 μM and 1 mM) it seemed to cause light tonic inward currents and then inhibited pacemaker amplitude and pacemaker frequency, and also an increase in the resting currents in the outward direction. Glibenclamide or other potassium channel blockers (TEA, BaCl2, apamin or 4-aminopydirine) did not have an effect on NaHS-induced action in ICC. The exogenous application of carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP) and thapsigargin also inhibited the pacemaker activity of ICC as NaHS. Also, we found NaHS inhibited the spontaneous intracellular Ca2+ ([Ca2+]i) oscillations in cultured ICC. In doing an RT-PCR experiment, we found that ICC enriched population lacked mRNA for both CSE and CBS, but was prominently detected in unsorted muscle. In conclusion, H2S inhibited the pacemaker activity of ICC by modulating intracellular Ca2+. These results can serve as evidence of the physiological action of H2S as acting on the ICC in gastrointestinal (GI) motility.
Go to : 
References
1. Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia NW, Reiffenstein RJ, Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: post-mortem studies and two case reports. J Anal Toxicol. 1989; 13:105–109.

2. Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, Dicken FP. Acute hydrogen sulfide poisoning: demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide level. Biochem Pharmacol. 1989; 38:973–981.
3. Savage JC, Gould DH. Determination of sulfides in brain tissue and rumen fluid by ion-interaction reversed-phased high-performance liquid chromatography. J Chromatogr. 1990; 526:540–545.
4. Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol. 2004; 142:31–34.
5. Hosoki R, Matsiki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophysic Res Commun. 1997; 237:527–541.

6. Gallego D, Clave P, Donovan J, Rahmati R, Grundy D, Jimenez M, Beyak MJ. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008; 20:1306–1316.
7. Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995; 269:C1577–1588.

8. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001; 20:6008–6016.
9. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996; 16:1066–1071.

10. Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Ann Rev Pharmacol Toxicol. 1992; 32:109–134.

12. Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of Kir6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006; 18:187–198.
13. Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution of dual modulation of vascular tension. Toxicol. 2007; 232:138–146.
14. Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001; 533:155–163.

15. Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005; 144:1126–1137.
16. Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000; 525:105–111.

17. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525:355–361.

18. Jun JY, Choi S, Yeum CH, Chang IY, You HJ, Park CK, Kim MY, Kong ID, Kim MJ, Lee KP, So I, Kim KW. Substance P induces inward current and regulates pacemaker currents through tachykinin NK1 receptor in cultured interstitial cells of Cajal of murine small intestine. Eur J Pharmacol. 2004; 495:35–42.

19. Bukovska G, Kery V, Kraus JP. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Exp Purif. 1994; 5:442–448.
20. Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990; 269:335–340.
21. Barber T, Triguero A, Martinez LI, Torres L, Garcia C, Miralles VJ, Vina JR. Elevated expression of liver gamma-cystathionase is required for the maintenance of lactation in rats. J Nutr. 1999; 129:928–933.
Go to : 
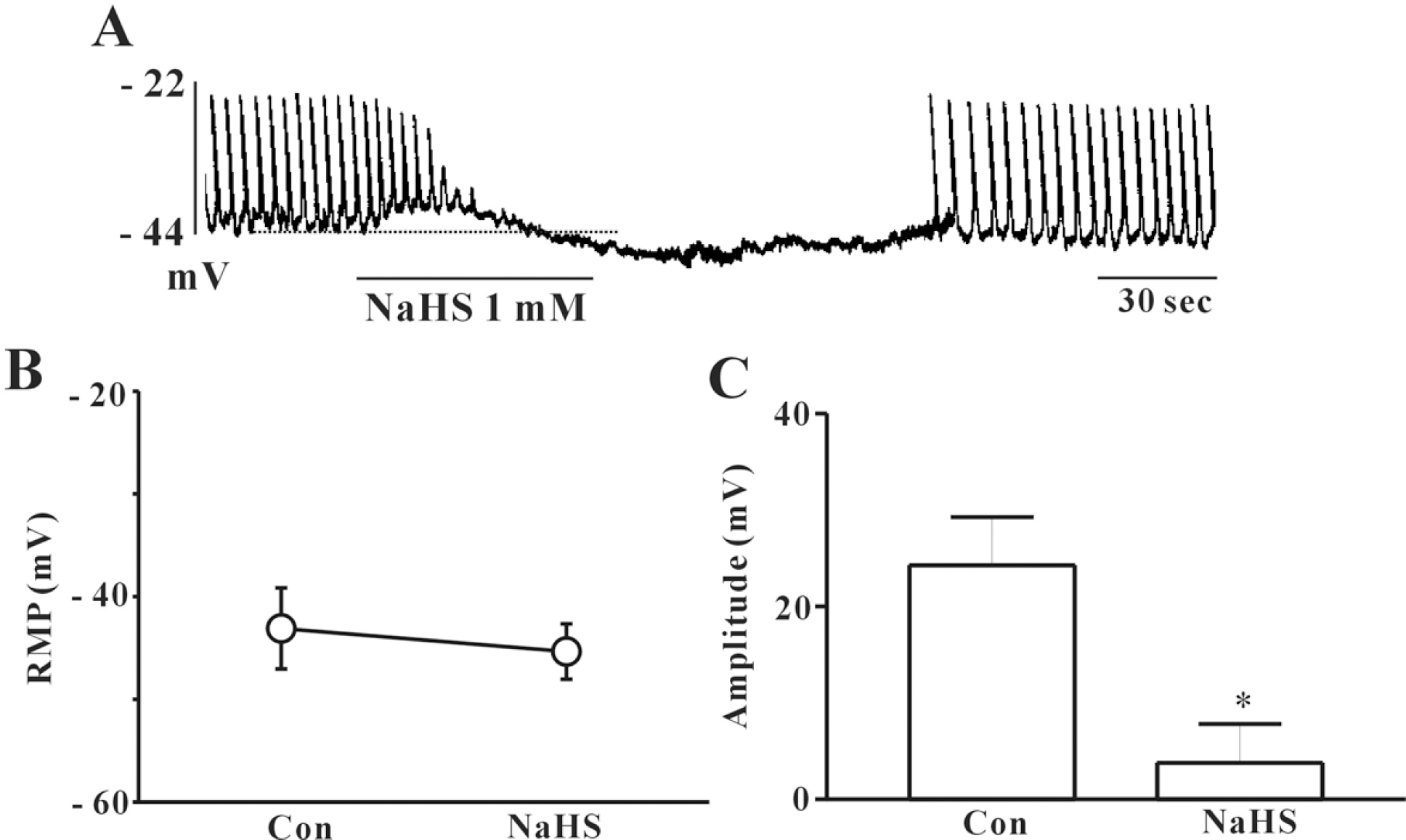 | Fig. 1.Effects of NaHS on pacemaker potentials recorded in cultured ICC from mouse small intestine. (A) shows the pacemaker potentials of ICC exposed to NaHS (1 mM) in the current clamping mode (I=0). NaHS induced membrane hyperpolarization and inhibited the amplitude and frequency of pacemaker potential in ICC. The dot lines indicate the control resting membrane potentials levels. Responses to NaHS are summarized in (B) and (C). The bars represent means±SE. ∗Asterisks mean significantly different from the controls (p<0.05). |
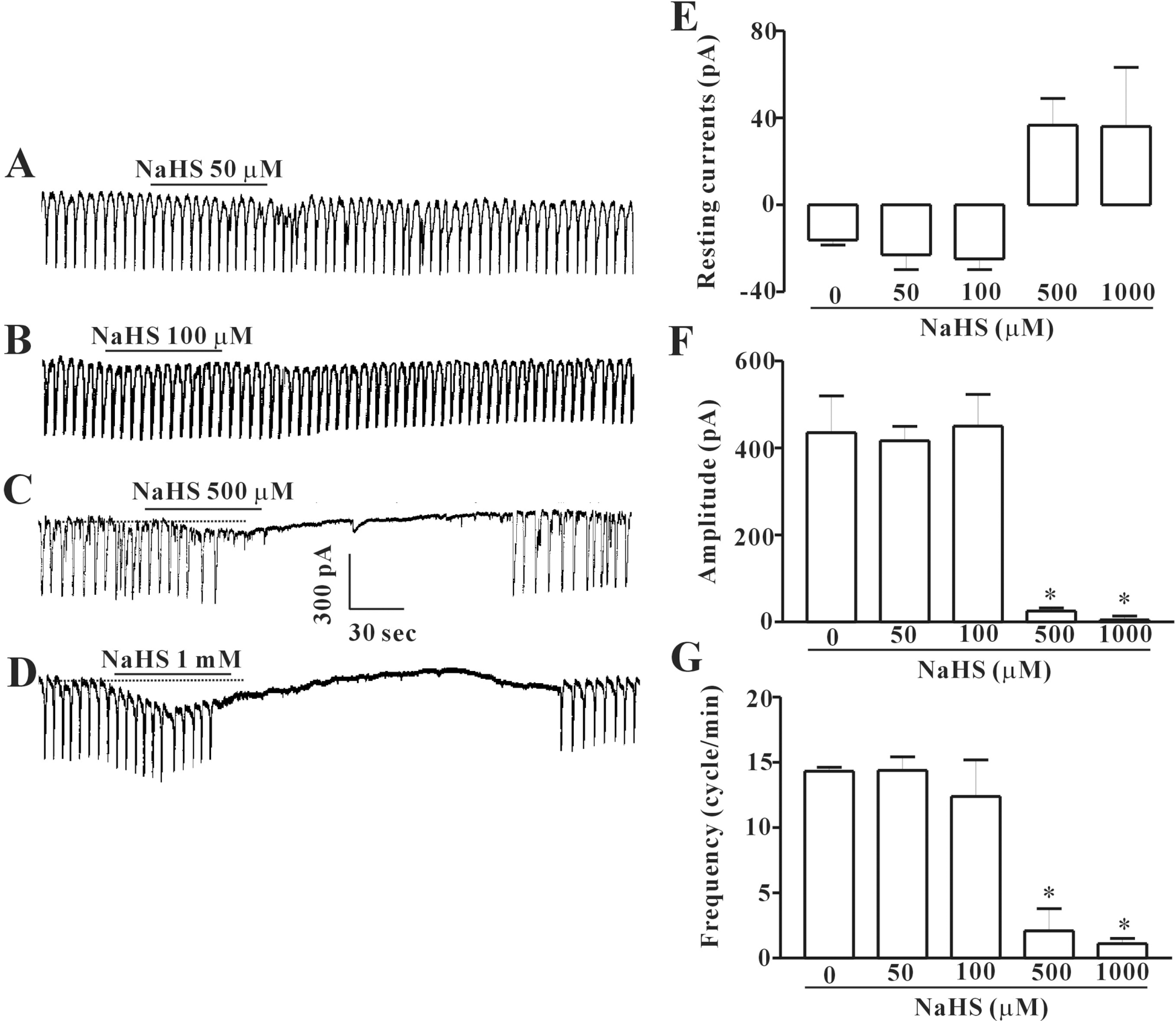 | Fig. 2.Effects of NaHS on pacemaker currents recorded in cultured ICC from mouse small intestine. (A∼C), and (D) show pacemaker currents of ICC exposed to NaHS (50, 100, 500 μM or 1 mM respectively) at a holding potential of – 70 mV. Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. The dot lines indicate the control resting current levels. (E, F), and (G) summarize the inhibitory effects of NaHS on pacemaker currents in ICC. Bars represent means±SE. ∗Asterisks mean significantly different from the controls (p<0.05). |
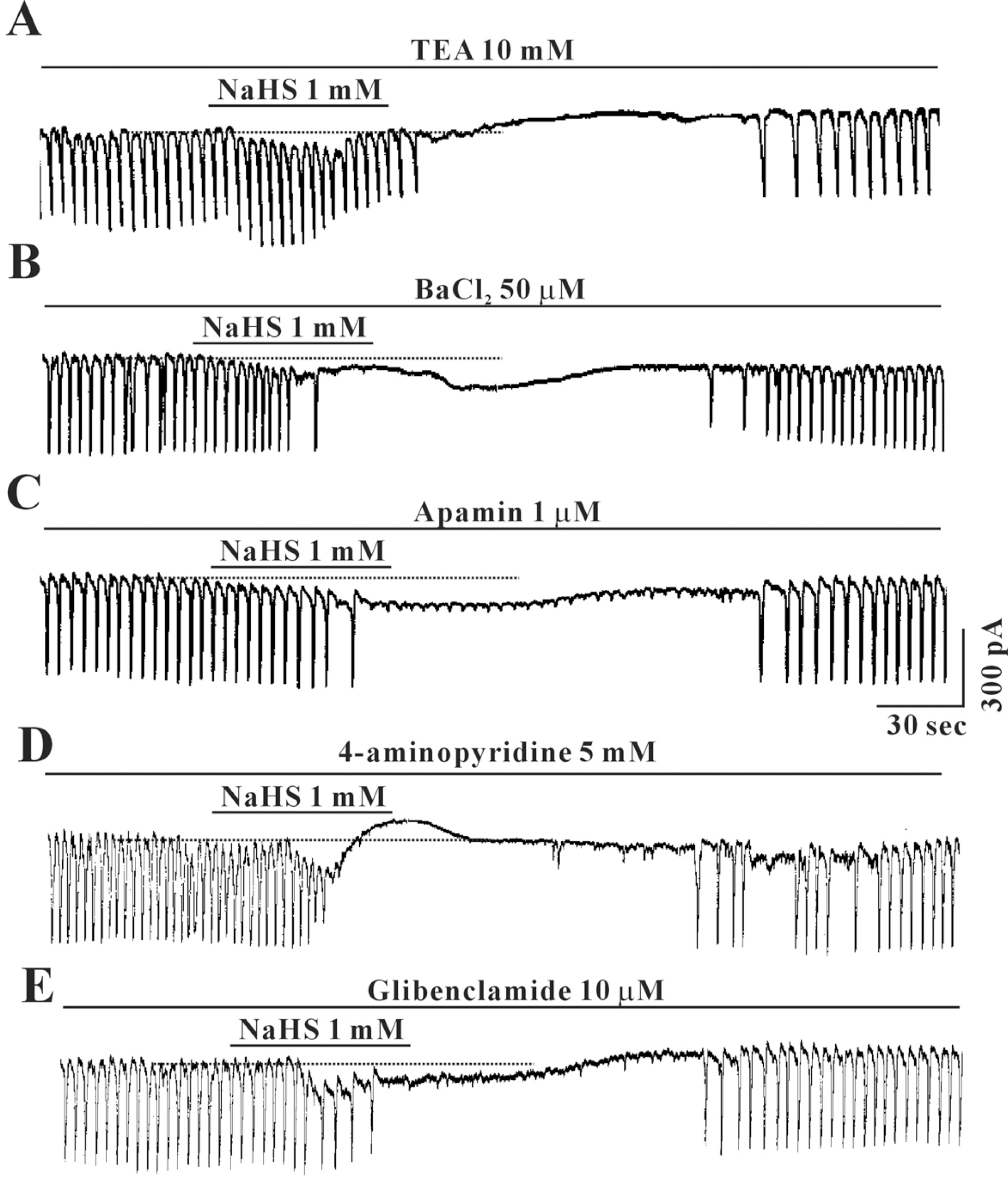 | Fig. 3.Effects of TEA, BaCl2, apamin, 4-aminopyridine and glibenclamide on NaHS-induced response in cultured ICC from mouse small intestine. Pretreatment with (A) TEA (20 mM), (B) BaCl2 (50 μM), (C) apamin (100 μM), (D) 4-aminopyridine (5 mM) or (E) glibenclamide (10 μM) did not affect the inhibitory effects of NaHS (1 mM). Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. The dot lines indicate the control resting current levels. TEA: tetraethylammonium chloride. |
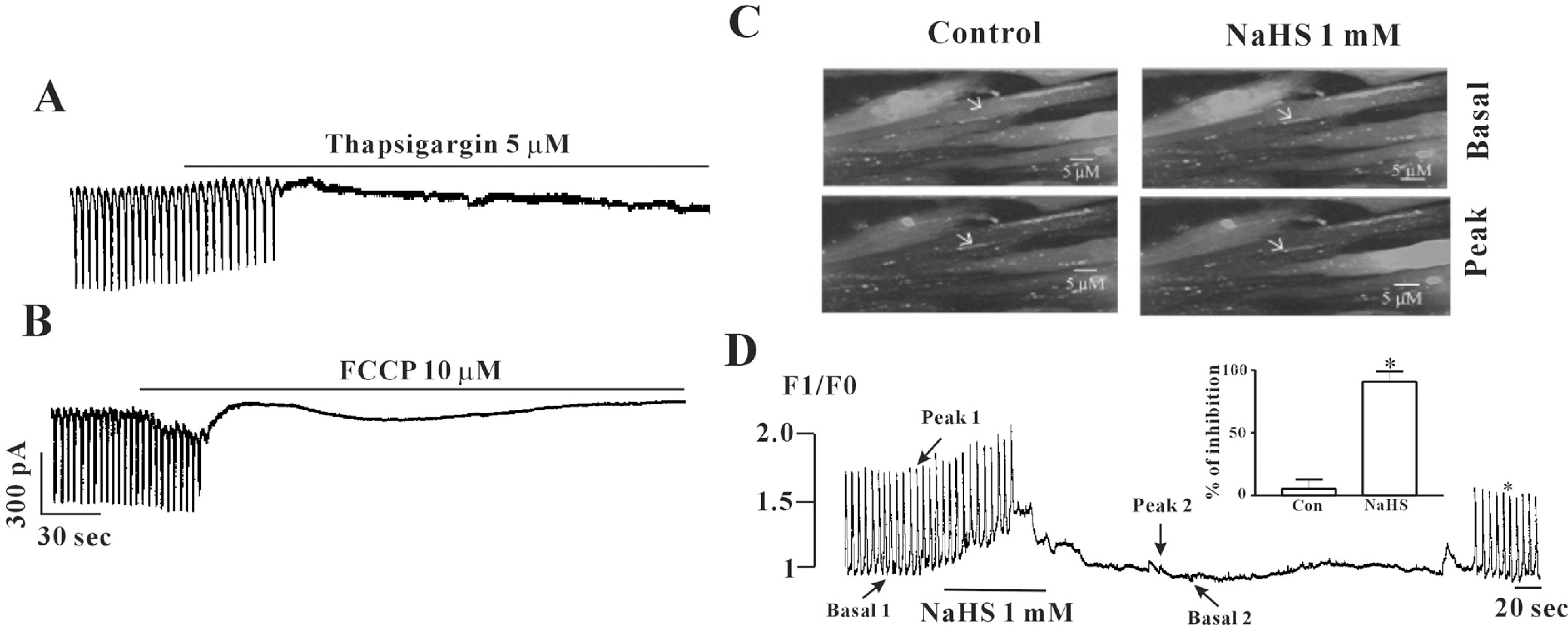 | Fig. 4.Effects of thapsigargin or FCCP on pacemaker currents and effects of NaHS on [Ca2+]i oscillation in cultured ICC from mouse small intestine. (A) Treatment with thapsigargin (5 μM) inhibited the pacemaker currents of ICC. (B) Treatment with FCCP (10 μM) in ICC also inhibited the pacemaker currents. (C) Sequential fluorescence intensity images of fluo-4-loaded cultured ICC in normal condition or in presence of NaHS (1 mM). The interval of representative frame was 1 second and the exposure time of each frame was 500 ms. (D) Fluorescence intensity changes plotted in (C) white marker. (Inset) Graphic representation of the inhibition of intensity at peak point of Ca2+ oscillations by NaHS. ∗(p<0.05) Significantly different from the untreated control. Con, control. |
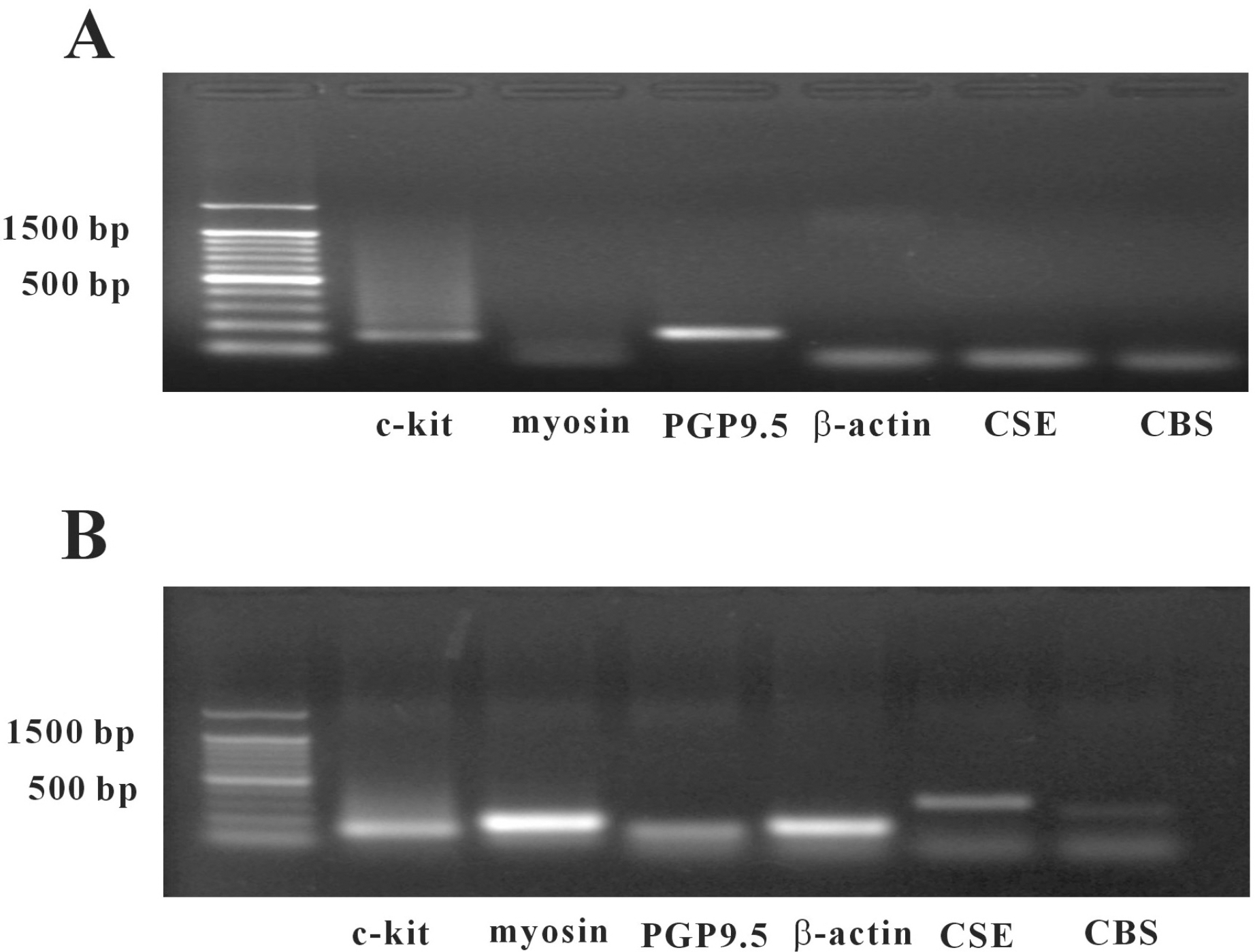 | Fig. 5.Agarose gels of the RT-PCR products of CSE and CBS enzymes using cultured cells and separated ICC. (A) Amplified cDNA prepared from pure ICC separated by magnetic cell separation and visualized in 2% gel. (B) Amplified cDNA from unsorted muscle cells and visualized in 2% gel (myosin-smooth muscle cells marker, PGP9.5-neuronal cell marker, CSE-cystathionine gama lyase, CBS-cystathionine beta synthase). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download