Abstract
Impairment in spinal inhibition caused by quantitative alteration of GABAergic elements following peripheral nerve injury has been postulated to mediate neuropathic pain. In the present study, we tested whether neuropathic pain could be induced or reversed by pharmacologically modulating spinal GABAergic activity, and whether quantitative alteration of spinal GABAergic elements after peripheral nerve injury was related to the impairment of GABAergic inhibition or neuropathic pain. To these aims, we first analyzed the pain behaviors following the spinal administration of GABA antagonists (1 μg bicuculline/rat and 5 μg phaclofen/rat), agonists (1 μg muscimol/rat and 0.5 μg baclofen/rat) or GABA transporter (GAT) inhibitors (20 μg NNC-711/rat and 1 μg SNAP-5114/rat) into naïve or neuropathic animals. Then, using Western blotting, PCR or immunohistochemistry, we compared the quantities of spinal GABA, its synthesizing enzymes (GAD65, 67) and its receptors (GABAA and GABAB) and transporters (GAT-1, and –3) between two groups of rats with different severity of neuropathic pain following partial injury of tail-innervating nerves; the allodynic and non-allodynic groups. Intrathecal administration of GABA antagonists markedly lowered tail-withdrawal threshold in naïve animals, and GABA agonists or GAT inhibitors significantly attenuated neuropathic pain in nerve-injured animals. However, any quantitative changes in spinal GABAergic elements were not observed in both the allodynic and non-allodynic groups. These results suggest that although the impairment in spinal GABAergic inhibition may play a role in mediation of neuropathic pain, it is not accomplished by the quantitative change in spinal elements for GABAergic inhibition and therefore these elements are not related to the generation of neuropathic pain following peripheral nerve injury.
Go to : 
References
1. Merskey H, Bogduk N. Classification of chronic pain. 2nd ed.Seattle: IASP Press;1994. p. 211.
2. Blumberg H, Janig W. Changes of reflexes in vasoconstrictor neurons supplying the cat hindlimb following chronic nerve lesions: a model for studying mechanisms of reflex sympathetic dystrophy? J Auton Nerv Syst. 1983; 7:399–411.

5. Boivie J. Central pain: Textbook of pain. Wall PD, Melzack R, editors. ed,. Textbook of pain. 3rd ed.New York: Churchill Livingstone;1994. p. 871–902.
6. Bonica JJ. Advances in Pain Research and Therapy. New York: Raven Press. 1979. 141–166.
7. Tasker RR. Pain resulting from central nervous system pathology (Central pain). Loeser JD, editor. ed,. The management of pain. 1st ed.Philadelphia: Lea and Febiger;1990. p. 264–284.
8. Back SK, Lee J, Hong SK, Na HS. Loss of spinal mu-opioid receptor is associated with mechanical allodynia in a rat model of peripheral neuropathy. Pain. 2006; 123:117–126.
9. Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004; 109:379–388.

10. Hao JX, Yu W, Xu XJ. Evidence that spinal endogenous opioidergic systems control the expression of chronic pain-related behaviors in spinally injured rats. Exp Brain Res. 1998; 118:259–268.

11. Na HS, Kim HJ, Sung B, Back SK, Kim DY, Kim JS, Hong SK. Decrease in spinal CGRP and substance P is not related to neuropathic pain in a rat model. Neuroreport. 2001; 12:175–178.

12. Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002; 22:6724–6731.

13. Patel S, Naeem S, Kesingl A, Froestl W, Capogna M, Urban L, Fox A. The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001; 90:217–226.

14. Somers DL, Clemente FR. Dorsal horn synaptosomal content of aspartate, glutamate, glycine and GABA are differentially altered following chronic constriction injury to the rat sciatic nerve. Neurosci Lett. 2002; 323:171–174.

15. Hao JX, Xu XJ, Wiesenfeld-Hallin Z. Intrathecal gamma-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett. 1994; 182:299–302.
16. Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002; 96:1161–1167.
17. Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994; 72:169–179.

18. Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989; 37:111–123.

19. Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993; 620:287–291.

20. Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998; 16:57–72.
21. Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997; 76:845–858.

22. Ralston DD BM, Sehlhorst SC, Meng X W, Ralston HJ. Decreased GABA immunoreactivity in rat dorsal horn is correlated with pain behavior:a light and electron microscopic study. Jensen TS, Turner JA, Wiesenfeld-Hallin Z, editors. ed,. Preoceedings of the Eighth World Congress on Pain. Seattle: WA, IASP Press;1997. p. 547–560.
23. Bhisitkul RB, Kocsis JD, Gordon TR, Waxman SG. Trophic influence of the distal nerve segment on GABAA receptor expression in axotomized adult sensory neurons. Exp Neurol. 1990; 109:273–278.

24. Castro-Lopes JM, Malcangio M, Pan BH, Bowery NG. Complex changes of GABAA and GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy. Brain Res. 1995; 679:289–297.

25. Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003; 105:347–353.

26. Engle MP, Gassman M, Sykes KT, Bettler B, Hammond DL. Spinal nerve ligation does not alter the expression or function of GABA(B) receptors in spinal cord and dorsal root ganglia of the rat. Neuroscience. 2006; 138:1277–1287.

27. Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004; 111:144–150.
28. Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005; 25:6658–6666.

29. Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003; 104:229–239.
30. Satoh O, Omote K. Roles of monoaminergic, glycinergic and GABAergic inhibitory systems in the spinal cord in rats with peripheral mononeuropathy. Brain Res. 1996; 728:27–36.

31. Kontinen VK, Stanfa LC, Basu A, Dickenson AH. Electrophysiologic evidence for increased endogenous gabaergic but not glycinergic inhibitory tone in the rat spinal nerve ligation model of neuropathy. Anesthesiology. 2001; 94:333–339.

32. Back SK, Kim JS, Hong SK, Na HS. Ascending pathways for mechanical allodynia in a rat model of neuropathic pain. Neuroreport. 2003; 14:1623–1626.

33. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.

34. Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988; 8:2123–2130.

35. Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991; 56:720–723.
36. Alvarez FJ, Taylor-Blake B, Fyffe RE, De Blas AL, Light AR. Distribution of immunoreactivity for the beta 2 and beta 3 subunits of the GABAA receptor in the mammalian spinal cord. J Comp Neurol. 1996; 365:392–412.
37. Ng CH, Ong WY. Increased expression of gamma-aminobutyric acid transporters GAT-1 and GAT-3 in the spinal trigeminal nucleus after facial carrageenan injections. Pain. 2001; 92:29–40.
38. Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997; 70:15–22.

39. Smith GD, Harrison SM, Birch PJ, Elliott PJ, Malcangio M, Bowery NG. Increased sensitivity to the antinociceptive activity of (+/–)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994; 33:1103–1108.

41. Coggeshall RE, Lekan HA, White FA, Woolf CJ. A-fiber sensory input induces neuronal cell death in the dorsal horn of the adult rat spinal cord. J Comp Neurol. 2001; 435:276–282.

42. Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003; 424:938–942.

43. Hu JH, Yang N, Ma YH, Zhou XG, Jiang J, Duan SH, Mei ZT, Fei J, Guo LH. Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice. J Neurosci Res. 2003; 73:565–572.
Go to : 
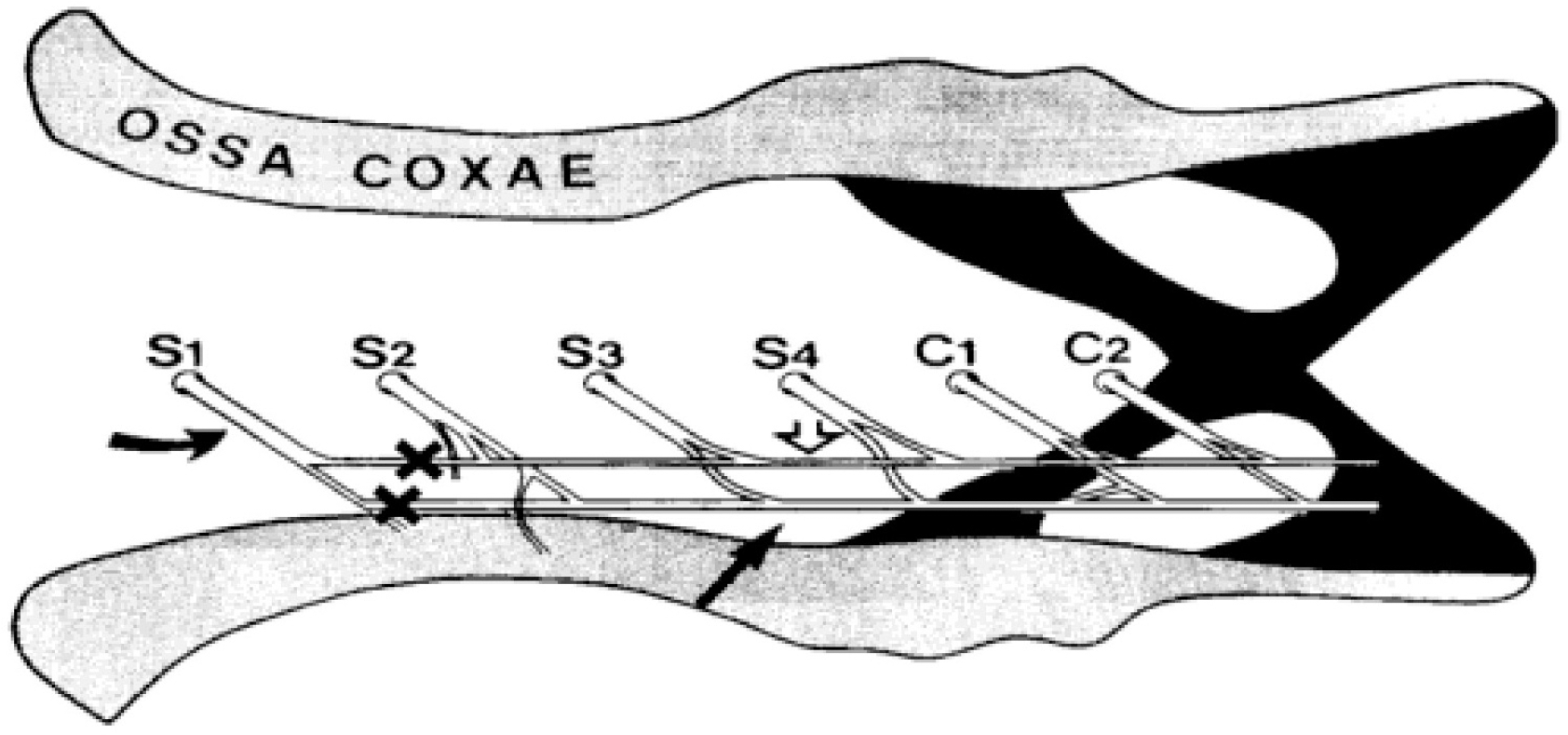 | Fig. 1.A schematic diagram illustrating how the inferior (black arrow) and superior (open arrow) caudal trunks are composed and the level of the transection (X) of nerve trunks. The curved arrow indicates the S1 spinal nerve. |
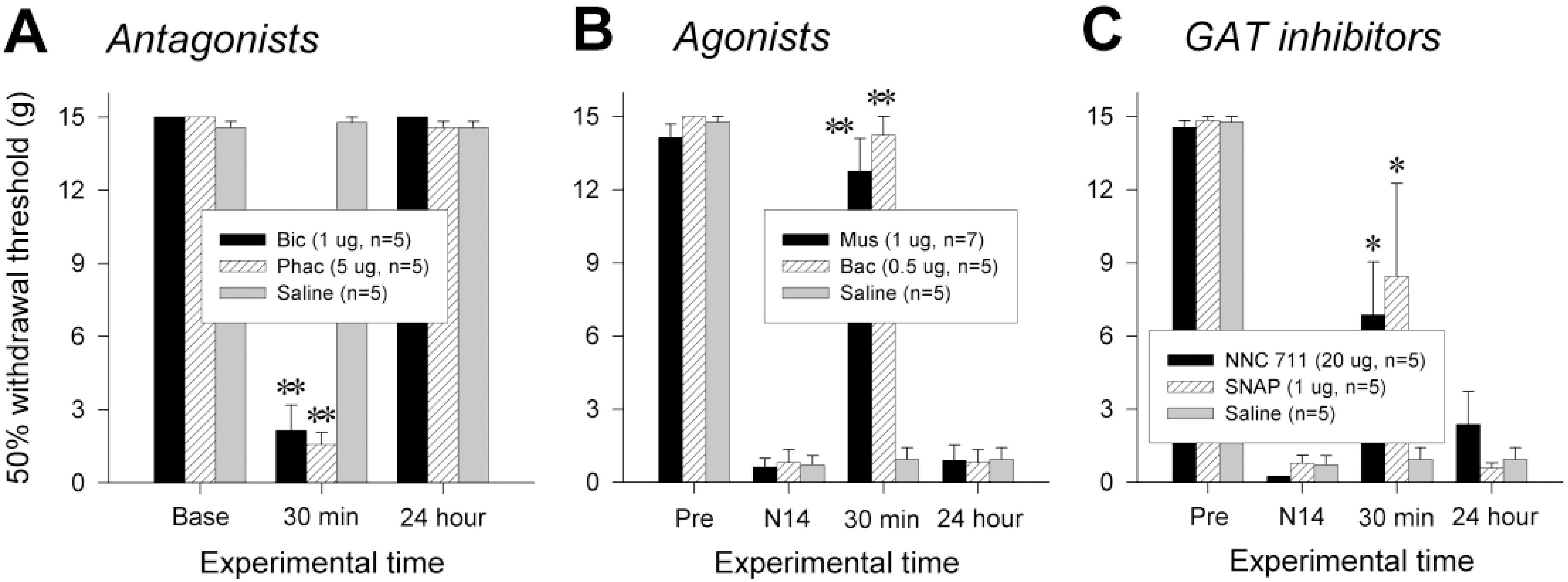 | Fig. 2.Changes in pain behaviors by pharmacological regulation of spinal GABAergic activity. (A) Mechanical allodynia induced by intrathecal administration of GABA antagonists, bicuculline and phaclofen, in naïve rats. (B) Attenuation of nerve injury-induced mechanical allodynia by GABA agonists, muscimol and baclofen, applied spinally. (C) Reversal of nerve injury-induced mechanical allodynia by intrathecally administered GAT inhibitors, NNC 711 for GAT-1 and SNAP 5114 for GAT-3. ‘Pre’ and ‘N14’ indicate 1 day before and 14 days after neuropathic surgery, respectively. ∗p≤0.05, ∗∗p≤0.001 vs. pre-injection value (One-way repeated measures ANOVA followed by Bonferroni t-test). |
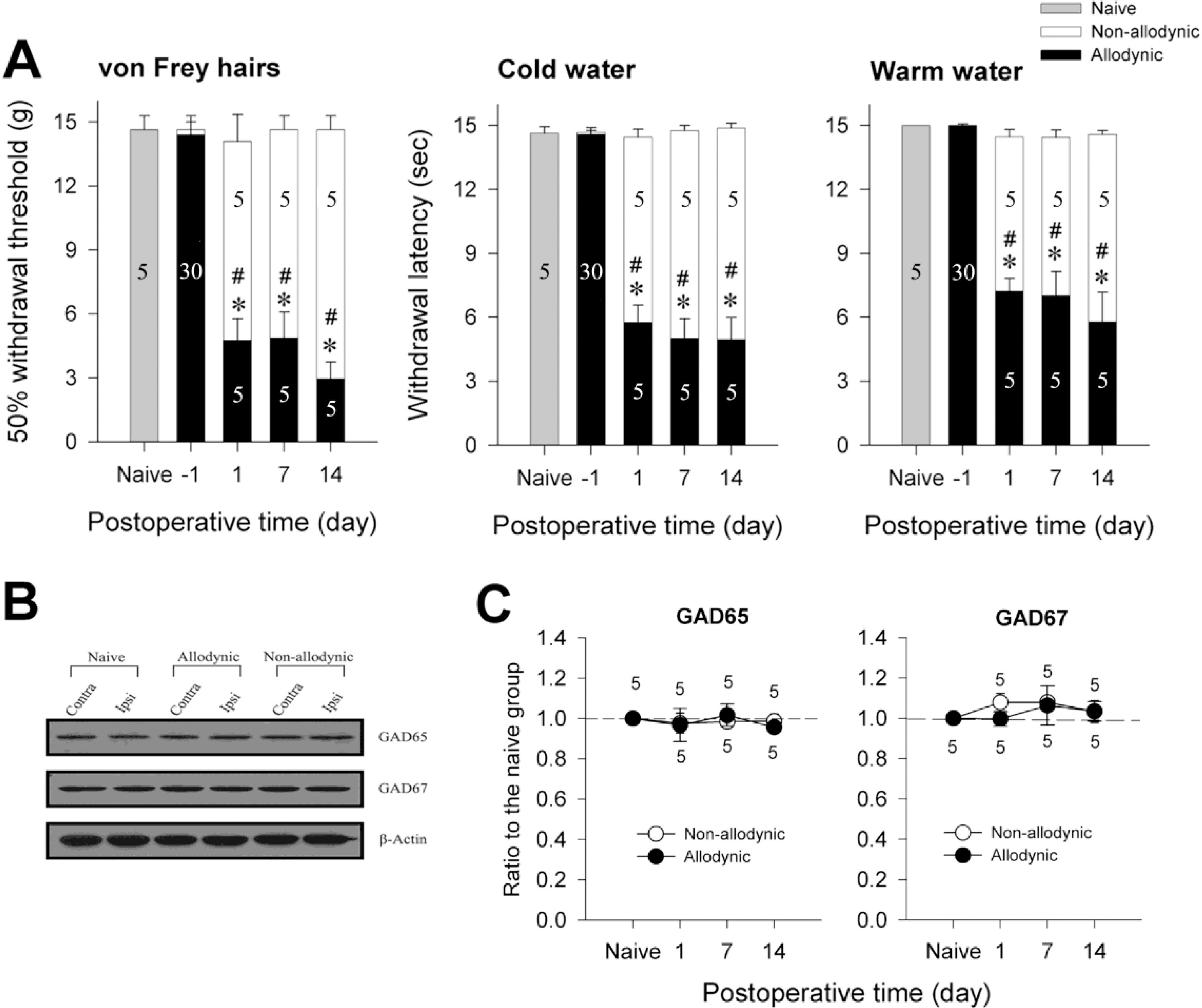 | Fig. 3.Western blot analysis for spinal GAD65 and GAD67 among the naïve, allodynic and non-allodynic animals. (A) Difference in the manifestation of neuropathic pain between the allodynic and non-allodynic groups following the same nerve injury, ∗p≤0.001 vs. the naïve group; #p≤0.001 vs. the non-allodynic group (One-way ANOVA followed by all pairwise multiple comparison, utilizing Holm-Sidak method). (B) Immunoblots of two isoforms of GAD extracted from the S1 spinal segments (injured level) 2 weeks following neuropathic surgery. (C) The temporal courses of immunoreactivities of GAD65 and GAD67 in the ipsilateral hemi-cords. Data are normalized to β-actin and expressed as a ratio to naïve. GAD65-and GAD67-ir in the ipsilateral hemicords of the allodynic and non-allodynic groups are not different from those of the naïve group on any postoperative day. |
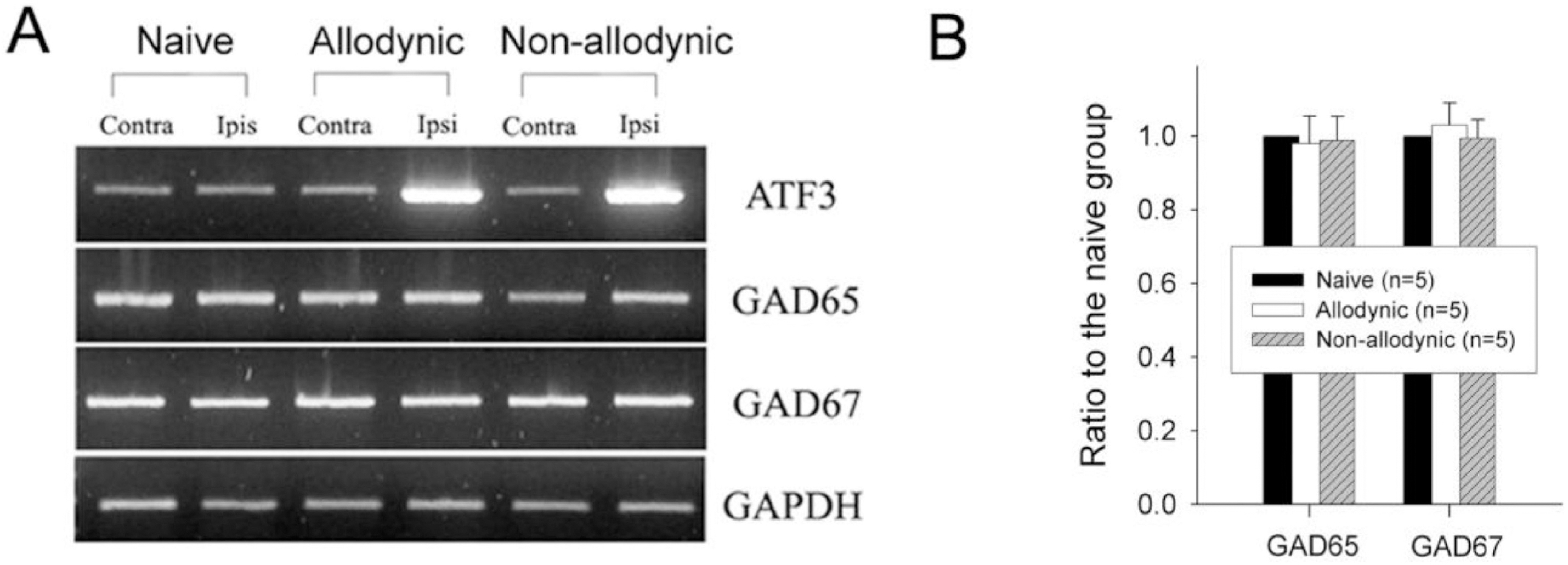 | Fig. 4.Reverse transcription-PCR analysis for GAD65 and GAD67 mRNA expression in the naïve, allodynic and non-allodynic groups 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 3A. (A) GAD65 and GAD 67 mRNA expression in the S1 spinal segments excised from the naïve, allodynic and non-allodynic animals. ATF3 mRNA expression in the S1 DRG was adopted as a marker of nerve injury. (B) Ipsilateral expression of GAD65 and GAD67 mRNA. Data are normalized to GAPDH and expressed as a ratio to naïve. Expressions of GAD65- and GAD67 mRNA in the ipsilateral hemi-cords of the allodynic and non-allodynic groups are not different from those of the naïve group. |
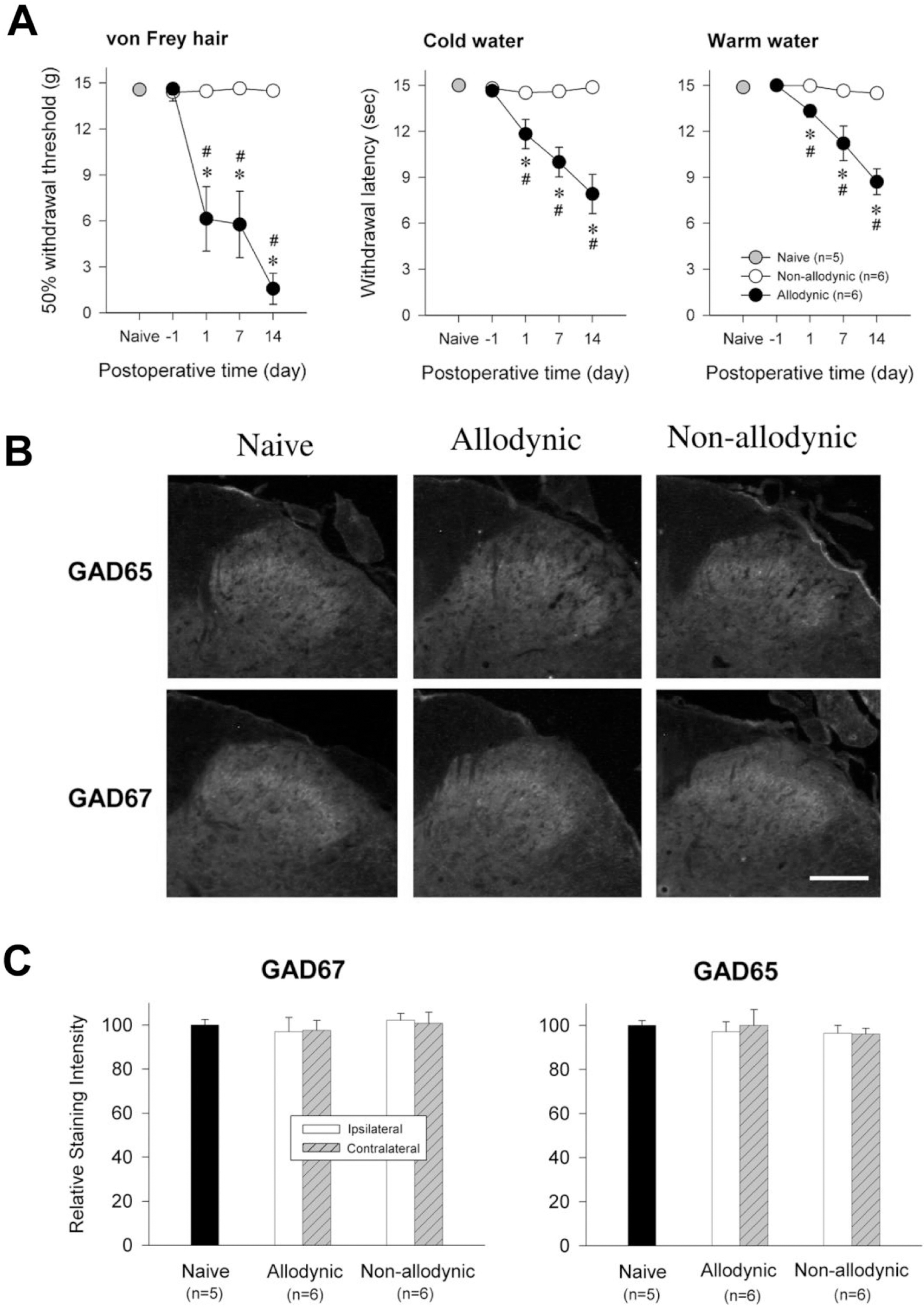 | Fig. 5.Immunohistochemial analysis for spinal GAD65 and GAD67 among the naïve, allodynic and non-allodynic animals 2 weeks following neuropathic surgery. (A) Difference in the manifestation of neuropathic pain between the allodynic and non- all-odynic groups, ∗p<0.05 vs. Pre-surgical value (One-way repeated ANOVA followed by Bonferroni t-test); #p<0.05 vs. non-allodynic group (Unpaired t-test). (B) Microphotographs illustrating distribution of GAD65- and GAD67-ir in the S1 spinal segment ipsilateral to nerve injury. Scale bar=100 μm. (C) Relative staining intensities of GAD65 and GAD67, compared to the naïve group. GAD65- and GAD67-ir in both the allodynic and non-allodynic groups are not different from those of the naïve group. |
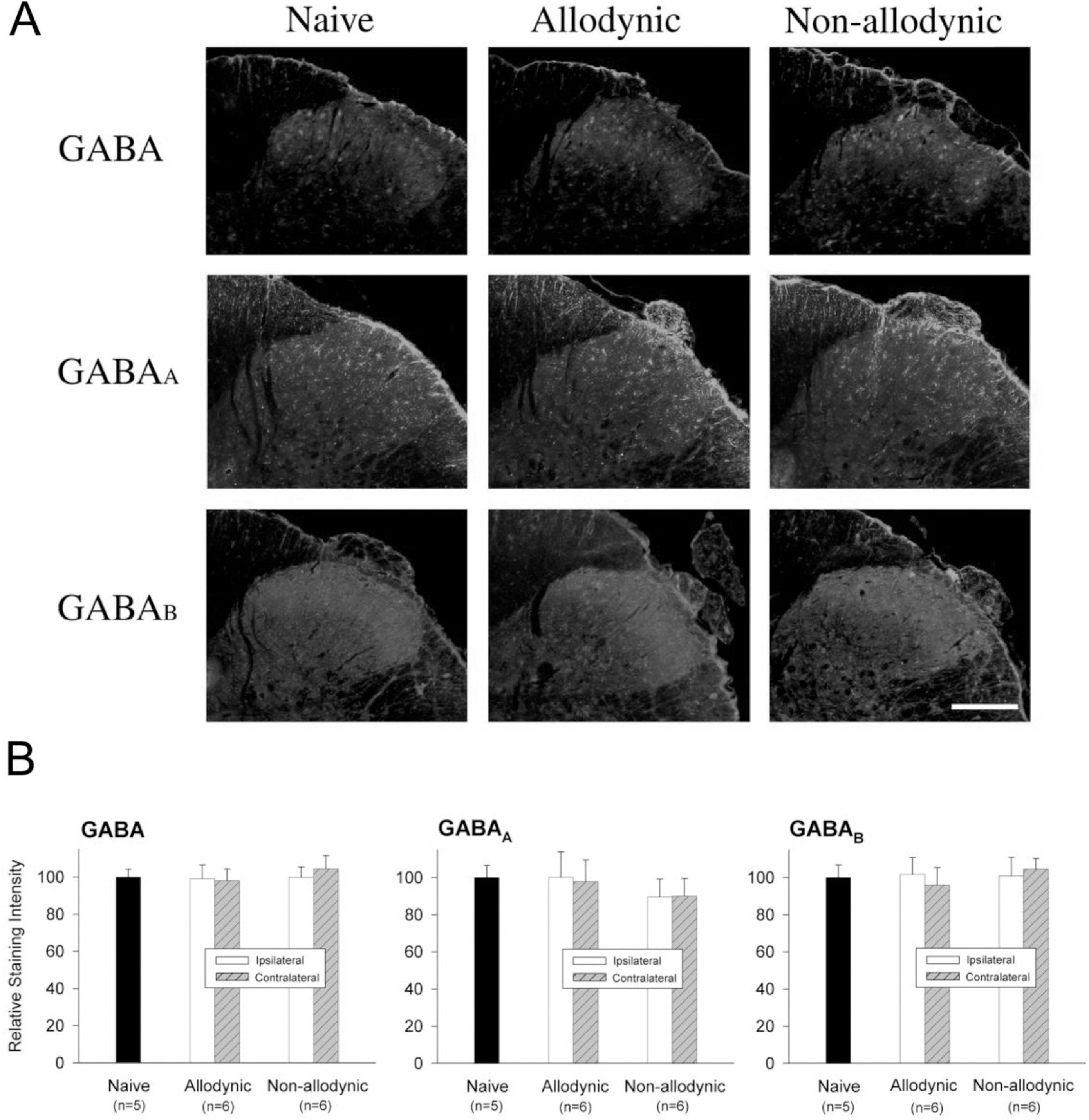 | Fig. 6.Immunohistochemial analysis for spinal GABA and its receptors, GABAA and GABAB, among the naïve, allodynic and non-allodynic animals 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 5A. (A) Microphotographs illustrating distribution of GABA-, GABAA and GABAB-ir in the S1 spinal segment ipsilateral to nerve injury. Scale bar=100 μm. (B) Relative staining intensities of GABA, GABAA and GABAB, compared to the naïve group. Immunoreactivities in both the all-odynic and non-allodynic groups are not different from those of the naïve group. |
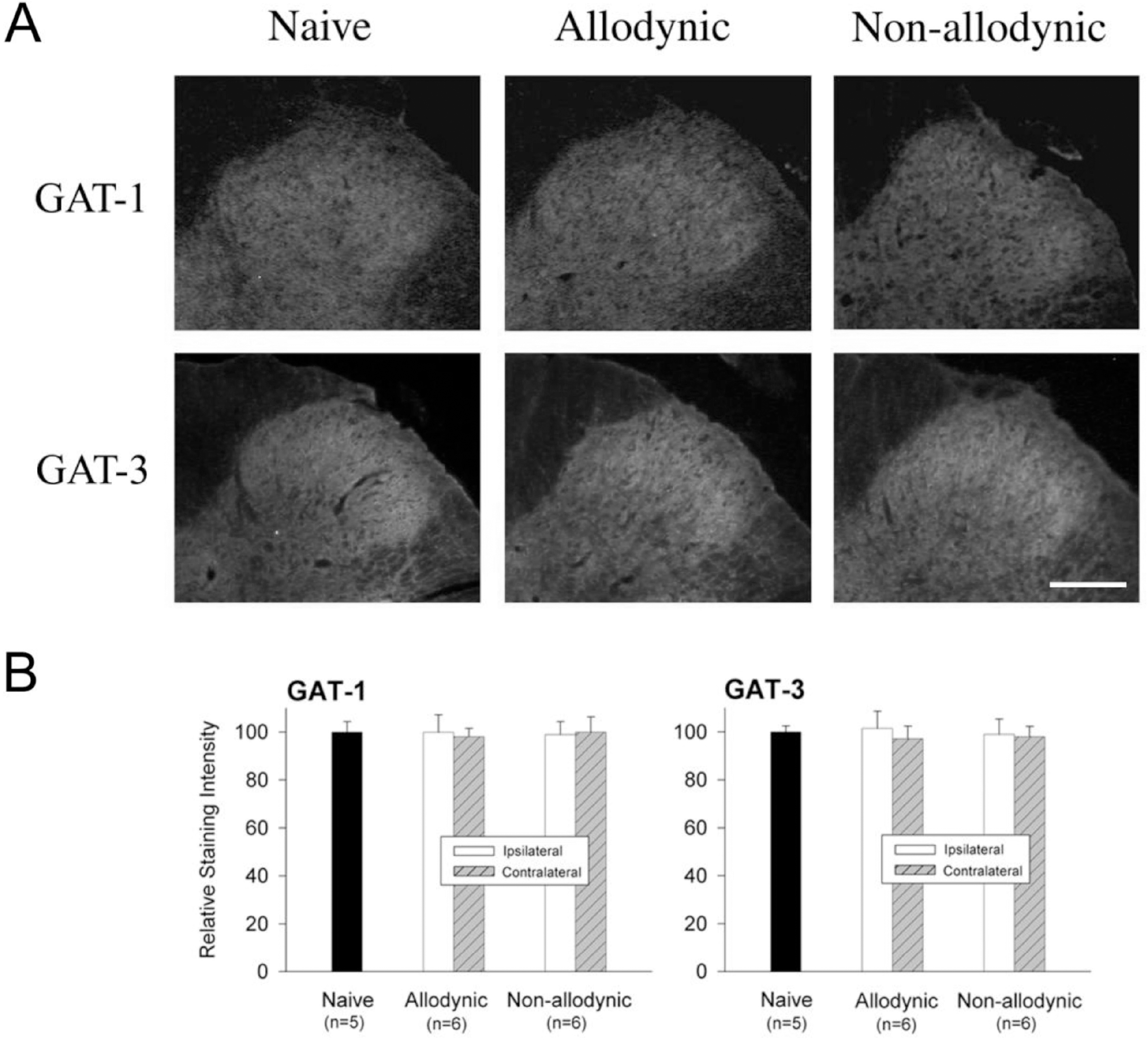 | Fig. 7.Immunohistochemial analysis for spinal GABA transporters, GAT-1 and GAT-3 among the naïve, all-odynic and non-allodynic animals 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 5A. (A) Microphotographs illustrating distribution of GAT-1- and GAT-3-ir in the S1 spinal segment ipsilateral to nerve injury. Scale bar=100 μm. (B) Relative staining intensities of GAT-1-and GAT-3 as compared to the naïve animals. Immunoreactivities in both the allodynic and non-allodynic groups are not different from those of the naïve group. |
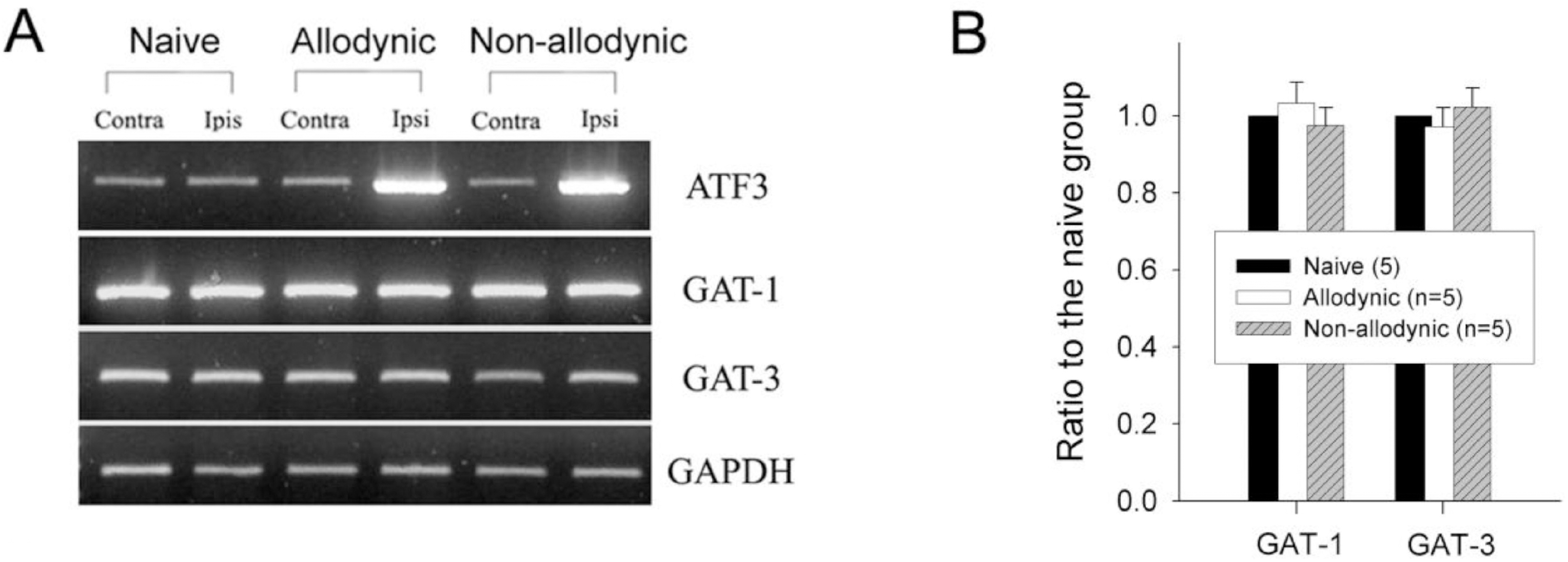 | Fig. 8.Reverse transcription-PCR analysis for GABA transporters, GAT-1 and GAT-3, mRNA expression in the naïve, allodynic and non-allodynic groups 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 3A. (A) GAT-1 and GAT-3 mRNA expressions in the S1 spinal segments excised from the naïve, allodynic and non-allodynic animals. ATF3 mRNA expression in the S1 DRG was adopted as a marker of nerve injury. (B) Ipsilateral expression of GAT-1 and GAT-3 mRNA. Data are normalized to GAPDH and expressed as a ratio to the naïve animals. Expressions of GAT-1 and GAT-3 mRNA in the ipsilateral hemi-cords of the allodynic and non-allodynic groups are not different from those of the naïve group. |
Table 1.
Sequences of primers used for RT-PCR
Table 2.
Postoperative changes in spinal levels of GABA synthesizing enzymes, GAD65 and GAD67. Data are expressed as percentage of naïve animals. POD indicates postoperative day. When compared with naïve animals, significant loss of spinal GAD65 or GAD67 was observed in neither ipsilateral nor contralateral side of both of the allodynic and non-allodynic groups at any postoperative day




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download