Abstract
This study was conducted to investigate the effects of extremely low frequency electromagnetic fields (EMF) on signal pathway in plasma membrane of cultured cells (RAW 264.7 cells and RBL 2H3 cells), by measuring the activity of phospholipase A2 (PLA2), phospholipase C (PLC) and phospholipase D (PLD). The cells were exposed to the EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. The basal and 0.5 μM melittin-induced arachidonic acid release was not affected by EMF in both cells. In cell-free PLA2 assay, we failed to observe the change of cPLA2 and sPLA2 activity. Also both PLC and PLD activities did not show any change in the two cell lines exposed to EMF. This study suggests that the exposure condition of EMF (60 Hz, 0.1 or 1 mT) which is 2.4 fold higher than the limit of occupational exposure does not induce phospholipases-associated signal pathway in RAW 264.7 cells and RBL 2H3 cells.
Go to : 
References
1. Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979; 109:273–284.

2. Floderus B, Persson T, Stenlund C, Wennberg A, Ost A, Knave B. Occupational exposure to electromagnetic fields in relation to leukemia and brain tumors: a case-control study in Sweden. Cancer Causes Control. 1993; 4:465–476.

3. Matanoski GM, Elliott EA, Breysse PN, Lynberg MC. Leukemia in telephone linemen. Am J Epidemiol. 1993; 137:609–619.

4. Glaser KB. Regulation of phospholipase A2 enzymes: selective inhibitors and their pharmacological potential. Adv Pharmacol. 1995; 32:31–66.
5. Attur MG, Patel R, Thakker G, Vyas P, Levartovsky D, Patel P, Naqvi S, Raza R, Patel K, Abramson D, Bruno G, Abramson SB, Amin AR. Differential anti-inflammatory effects of immunosuppressive drugs: cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE2 production. Inflamm Res. 2000; 49:20–26.

6. Petrone WF, English DK, Wong K, McCord JM. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980; 77:1159–1163.

7. Chilvers ER, Barnes PJ, Nahorski SR. Characterization of agonist-stimulated incorporation of myo-[3H]inositol into inositol phospholipids and [3H]inositol phosphate formation in tracheal smooth muscle. Biochem J. 1989; 262:739–746.
8. Claro E, Wallace MA, Lee HM, Fain JN. Carbachol in the presence of guanosine 5′-O-(3-thiotriphosphate) stimulates the breakdown of exogenous phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 4-phosphate, and phosphatidylinositol by rat brain membranes. J Biol Chem. 1989; 264:18288–18295.

9. Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984; 312:315–321.

10. Somlyo AV, Bond M, Somlyo AP, Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985; 82:5231–5235.

12. Billah MM, Anthes JC. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990; 269:281–291.

13. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992; 258:607–614.

14. Balsinde J, Barbour SE, Bianco ID, Dennis EA. Arachidonic acid mobilization in P388D1 macrophages is controlled by two distinct Ca2+-dependent phospholipase A2 enzymes. Proc Natl Acad Sci U S A. 1994; 91:11060–11064.
15. Martínez J, Moreno JJ. Role of Ca2+-Independent phospholipase A2 on arachidonic acid release Induced by reactive oxygen species. Arch Biochem Biophys. 2001; 392:257–262.
16. Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995; 270:445–450.
17. Fonteh AN, Bass DA, Marshall LA, Seeds M, Samet JM, Chilton FH. Evidence that secretory phospholipase A2 plays a role in arachidonic acid release and eicosanoid biosynthesis by mast cells. J Immunol. 1994; 152:5438–5446.
18. Dole VP, Meinertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960; 235:2595–2599.

19. Radvanyi Fi, Jordan L, Russo-Marie Fi, Bon C. A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal Biochem. 1989; 177:103–109.
20. Lee CW, Park DJ, Lee KH, Kim CG, Rhee SG. Purification, molecular cloning, and sequencing of phospholipase C-beta 4. J Biol Chem. 1993; 268:21318–21327.

21. Schmidt M, Hüwe SM, Fasselt B, Homann D, Rümenapp U, Sandmann J, Jakobs KH. Mechanisms of phospholipase D stimulation by m3 muscarinic acetylcholine receptors. Eur J Biochem. 1994; 225:667–675.
22. Da Silva A, Amrani Y, Trifilieff A, Landry Y. Involvement of B2 receptors in the bradykinin-induced relaxation of guinea-pig isolated trachea. Br J Pharmacol. 1995; 114:103–108.
23. Lusa S, Myllärniemi M, Volmonen K, Vauhkonen M, Somerharju P. Degradation of pyrene-labelled phospholipids by lysosomal phospholipases in vitro. Dependence of degradation on the length and position of the labelled and unlabelled acyl chains. Biochem J. 1996; 315:947–952.

24. Guidelines on limits of exposure to static magnetic fields. International Commission on Non-Ionizing Radiation Protection. Health Phys. 1994; 66:100–106.
25. Song HS, Park TW, Sohn UD, Shin YK, Choi BC, Kim CJ, Sim SS. The effect of caffeic acid on wound healing in skin-incised mice. Korean J Physiol Pharmacol. 2008; 12:343–347.

26. Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996; 1302:55–60.
27. Song HS, Park SH, Ko MS, Jeong JM, Sohn UD, Sim SS. Morinda citrifolia Inhibits both cytosolic Ca-dependent phospholipase A2 and secretory Ca-dependent phospholipase A2. Korean J Physiol Pharmacol. 2010; 14:163–167.
28. Patruno A, Amerio P, Pesce M, Vianale G, Di Luzio S, Tulli A, Franceschelli S, Grilli A, Muraro R, Reale M. Extremely low frequency electromagnetic fields modulate expression of inducible nitric oxide synthase, endothelial nitric oxide synthase and cyclooxygenase-2 in the human keratinocyte cell line HaCat: potential therapeutic effects in wound healing. Br J Dermatol. 2010; 162:258–266.

Go to : 
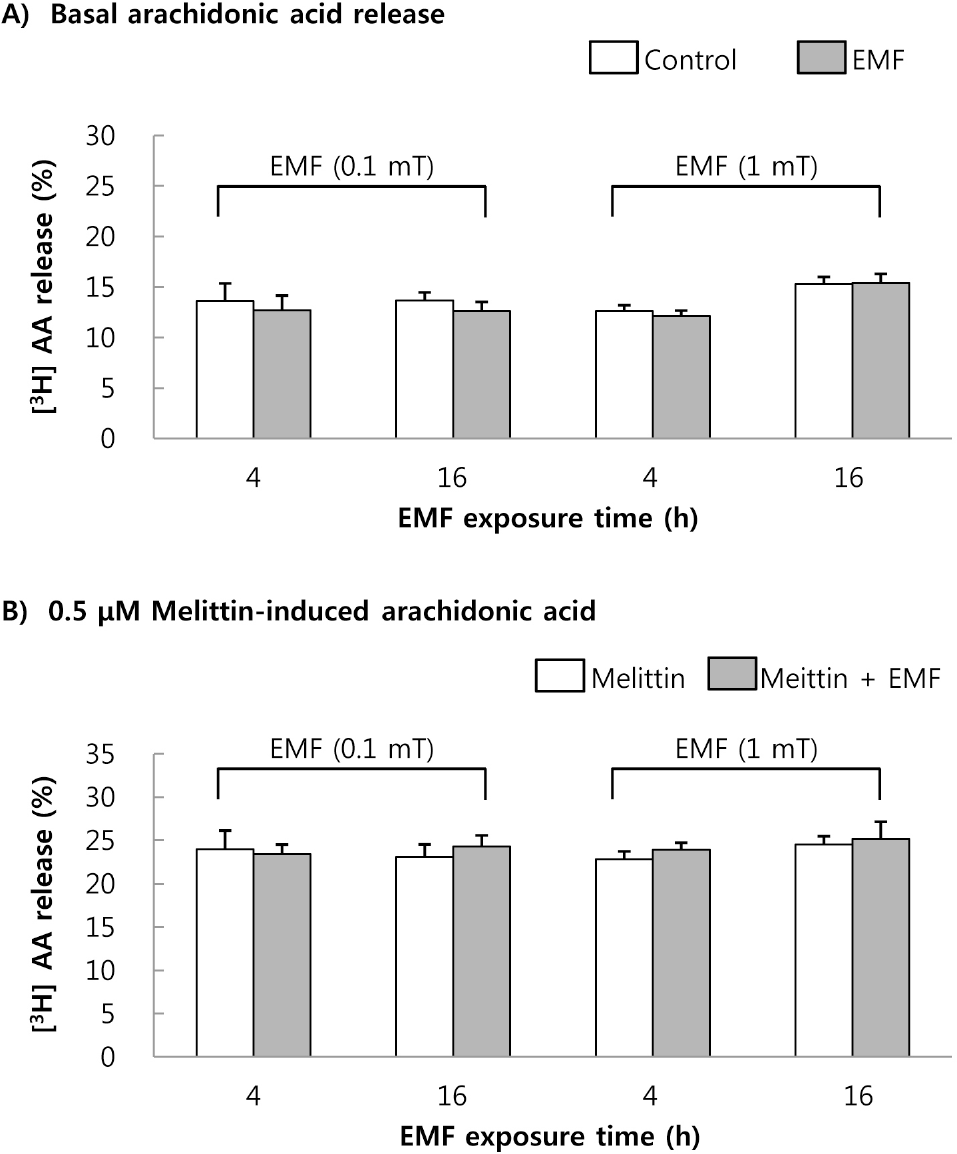 | Fig. 1.The changes of basal (A) and 0.5μM melittin-induced [3H]AA release (B) in RAW 264.7 cells. The cells were exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h and were labeled with [3H]AA for 2 h. The radioactivity of released [3H]AA was measured in the presence or absence of 0.5μM melittin. Results are indicated in mean±S.D. from four separate experiments. |
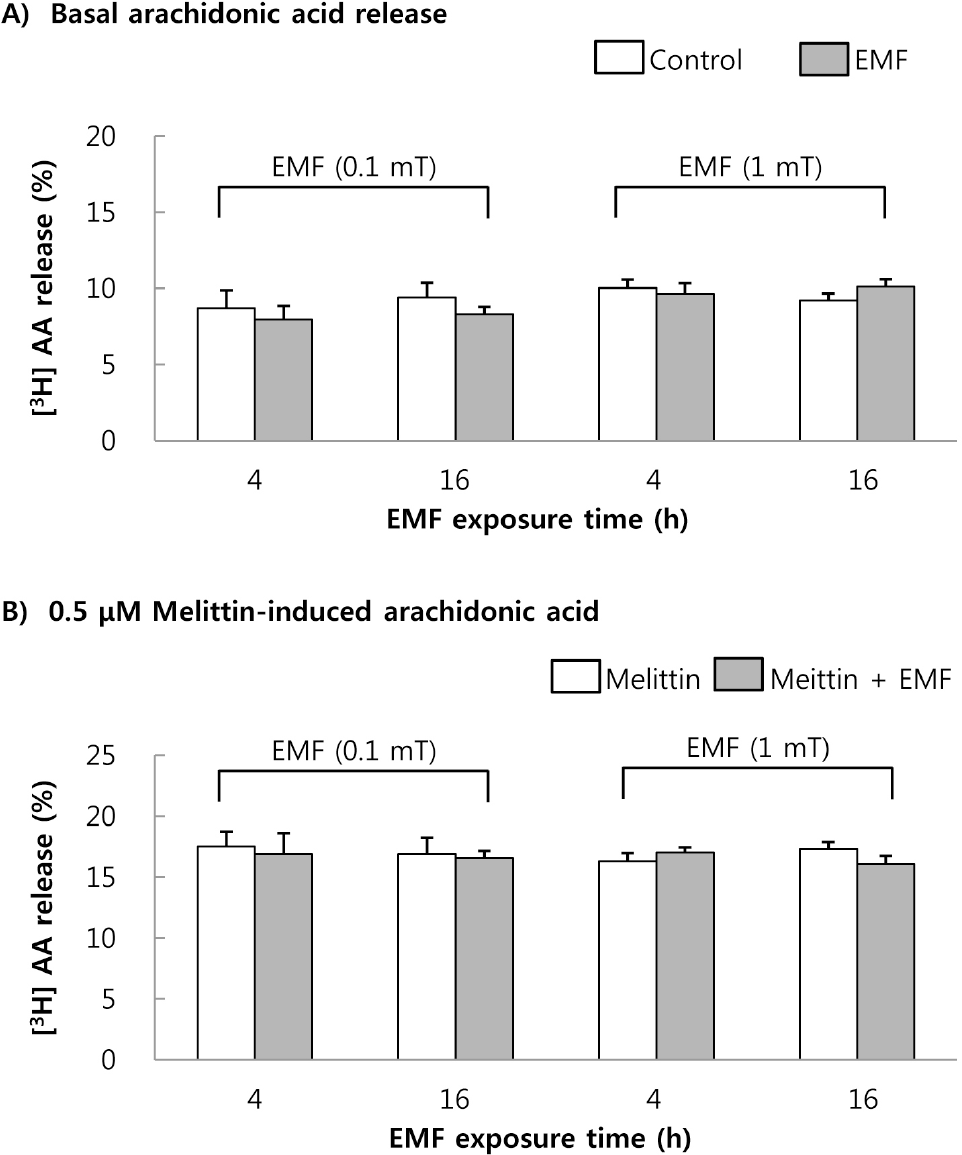 | Fig. 2.The changes of basal (A) and 0.5μM melittin-induced [3H]AA release (B) in RBL 2H3 cells. The cells were exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h and were labeled with [3H]AA for 2 h. The radioactivity of released [3H]AA was measured in the presence or absence of 0.5μM melittin. Results indicate mean±S.D. from four separate experiments. |
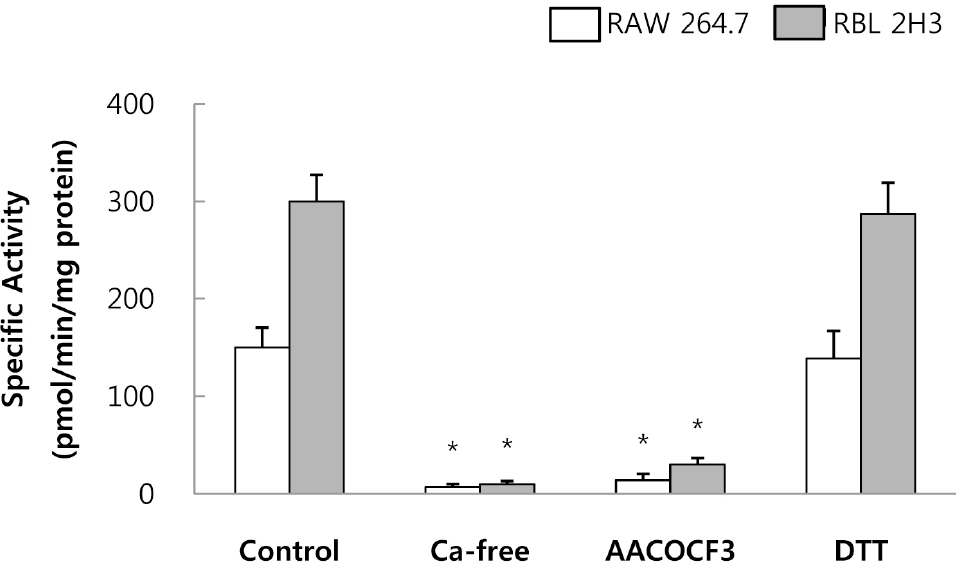 | Fig. 3.Cell-derived PLA2 activity in the presence of 5 mM CaCl2. PLA2 (25μg protein) derived from RAW 264.7 cells and RBL 2H3 cells was incubated with 1-palmitoyl-2-[14C]arachidonyl phosphatidylcholine in the presence of 10μM AACOCF3 (cPLA2 inhibitor) or 1 mM DTT (sPLA2 inhibitor) and in the absence of CaCl2. Results indicate mean±S.D. from four separate experiments. ∗Significantly different from Control (p<0.05). |
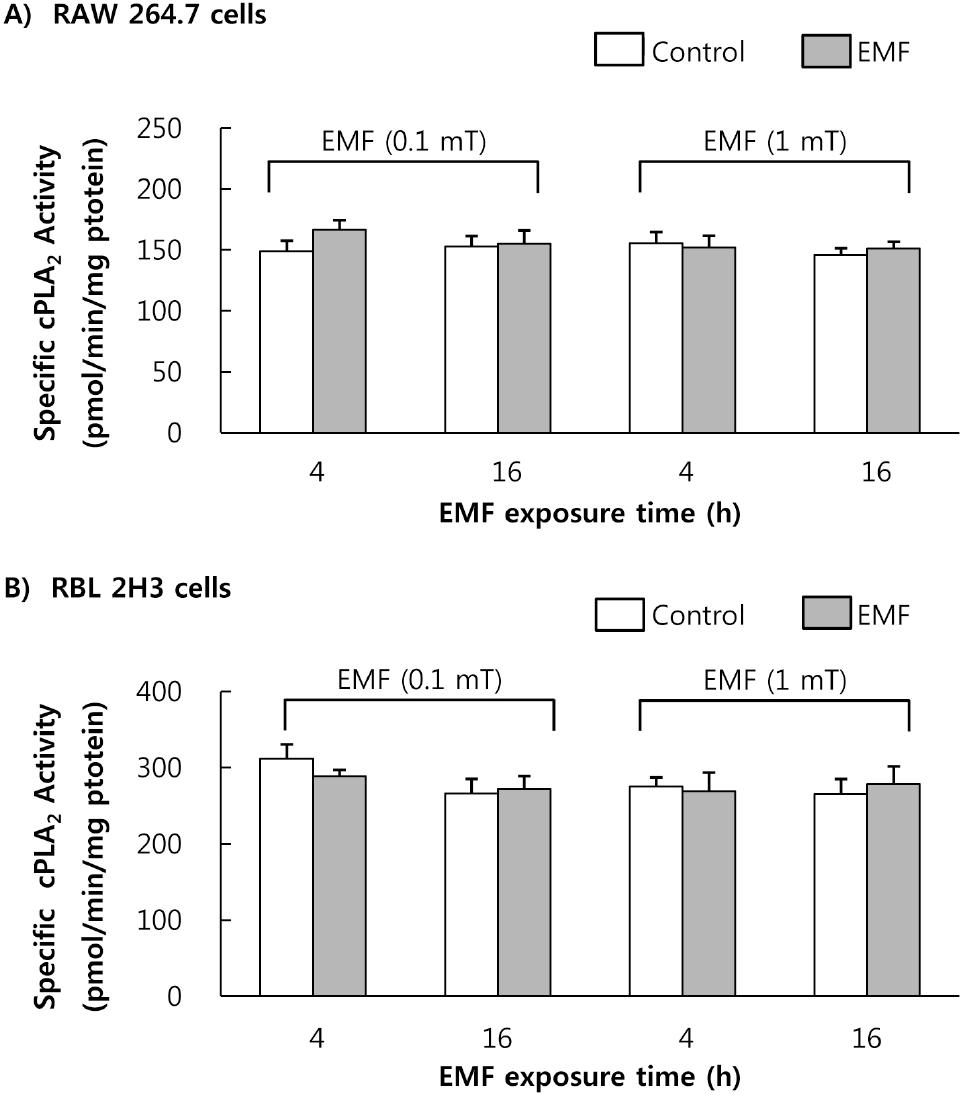 | Fig. 4.Effect of EMF on cell-derived cPLA2 activity. Cell-derived cPLA2 was obtained from the RAW 264.7 cells (A) and RBL 2H3 cells (B) exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. cPLA2 (25μg protein) was incubated with 1-palmitoyl-2-[14C]arachidonyl phosphatidylcholine in the presence of 5 mM CaCl2 and 1 mM DTT. Results indicate mean±S.D. from four separate experiments. |
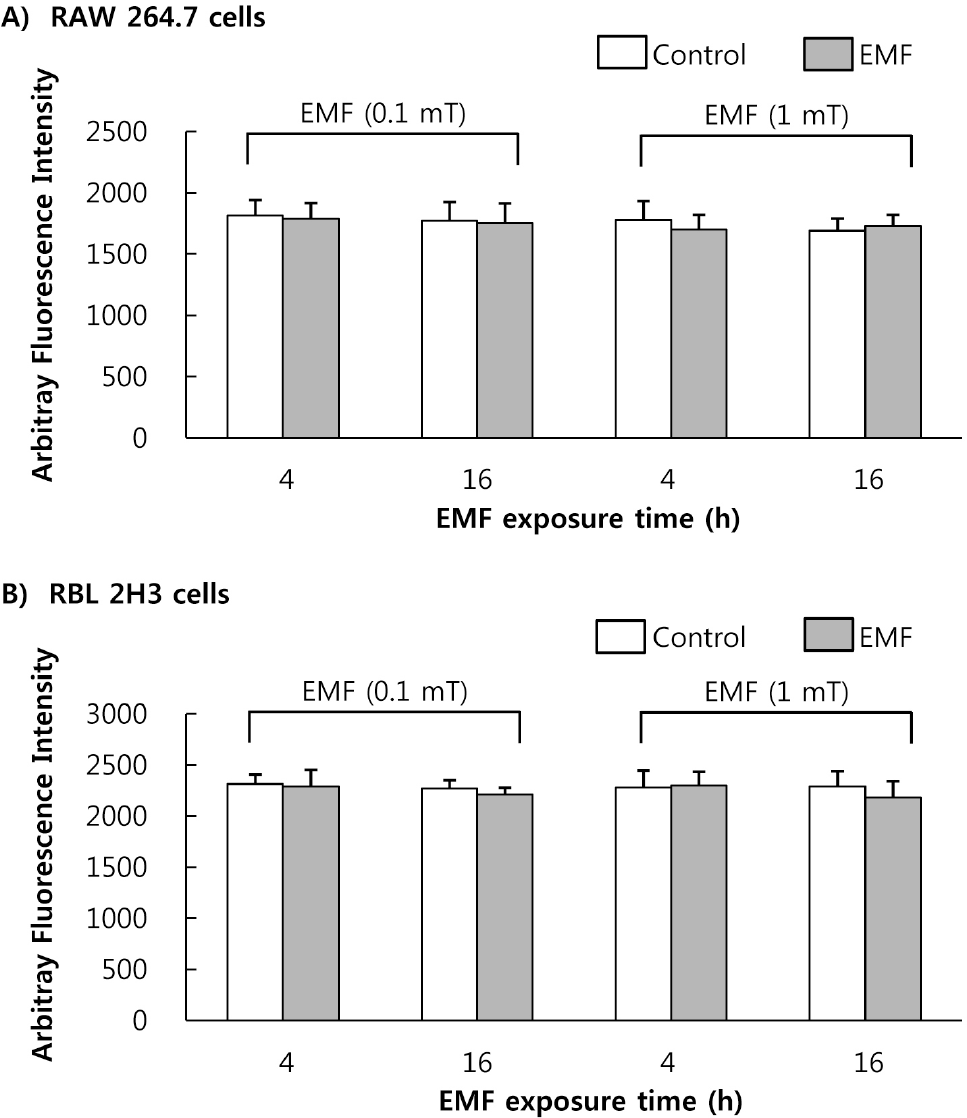 | Fig. 5.Effect of EMF on cell-derived sPLA2 activity. Cell-derived sPLA2 was obtained from the RAW 264.7 cells (A) and RBL 2H3 cells (B) exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. sPLA2 (100μg protein) was incubated with 10-pyren phosphatidylcholine. Results indicate mean±S.D. from four separate experiments. |
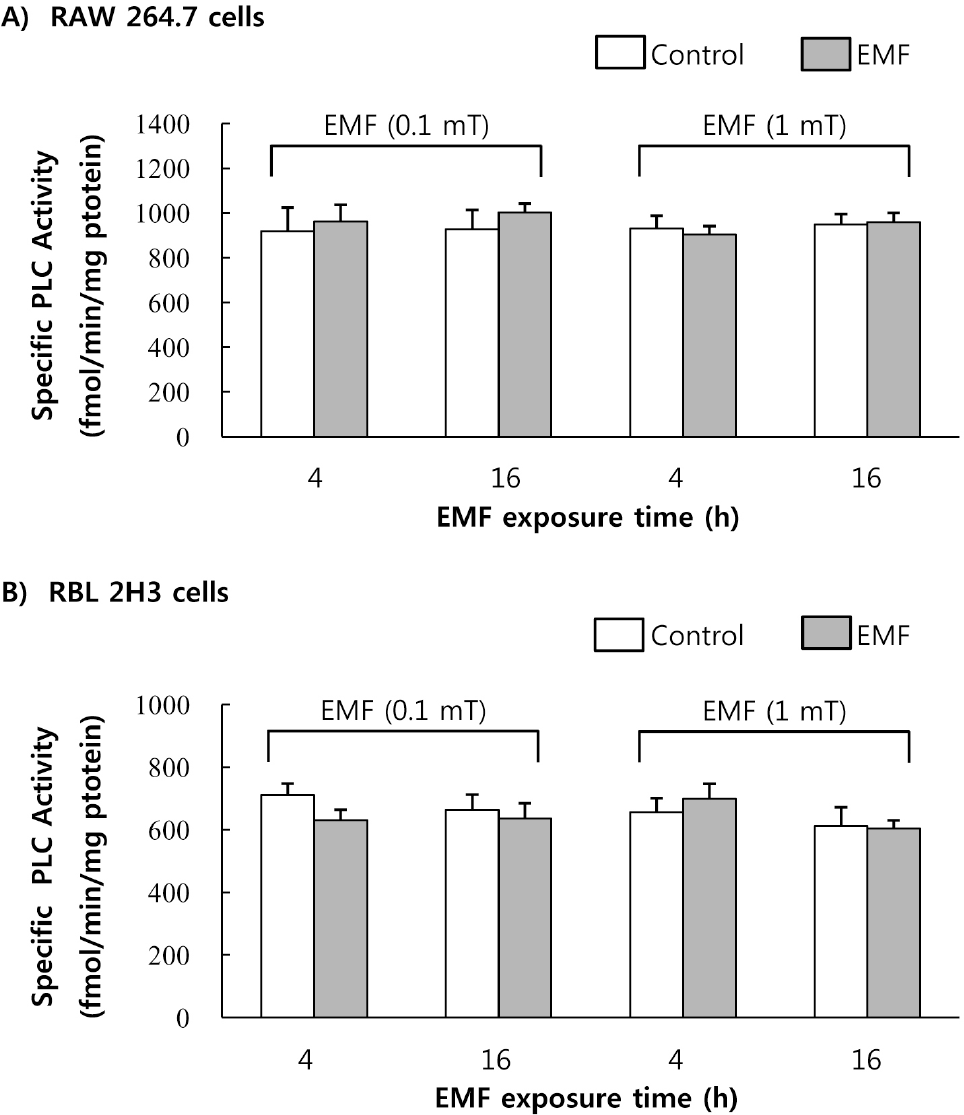 | Fig. 6.Effect of EMF on cell-derived PLC activity. Cell-derived PLC was obtained from the RAW 264.7 cells (A) and RBL 2H3 cells (B) exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. PLC (25μg protein) was incubated with [3H]phosphatidylinositol. Results indicate mean±S.D. from four separate experiments. |
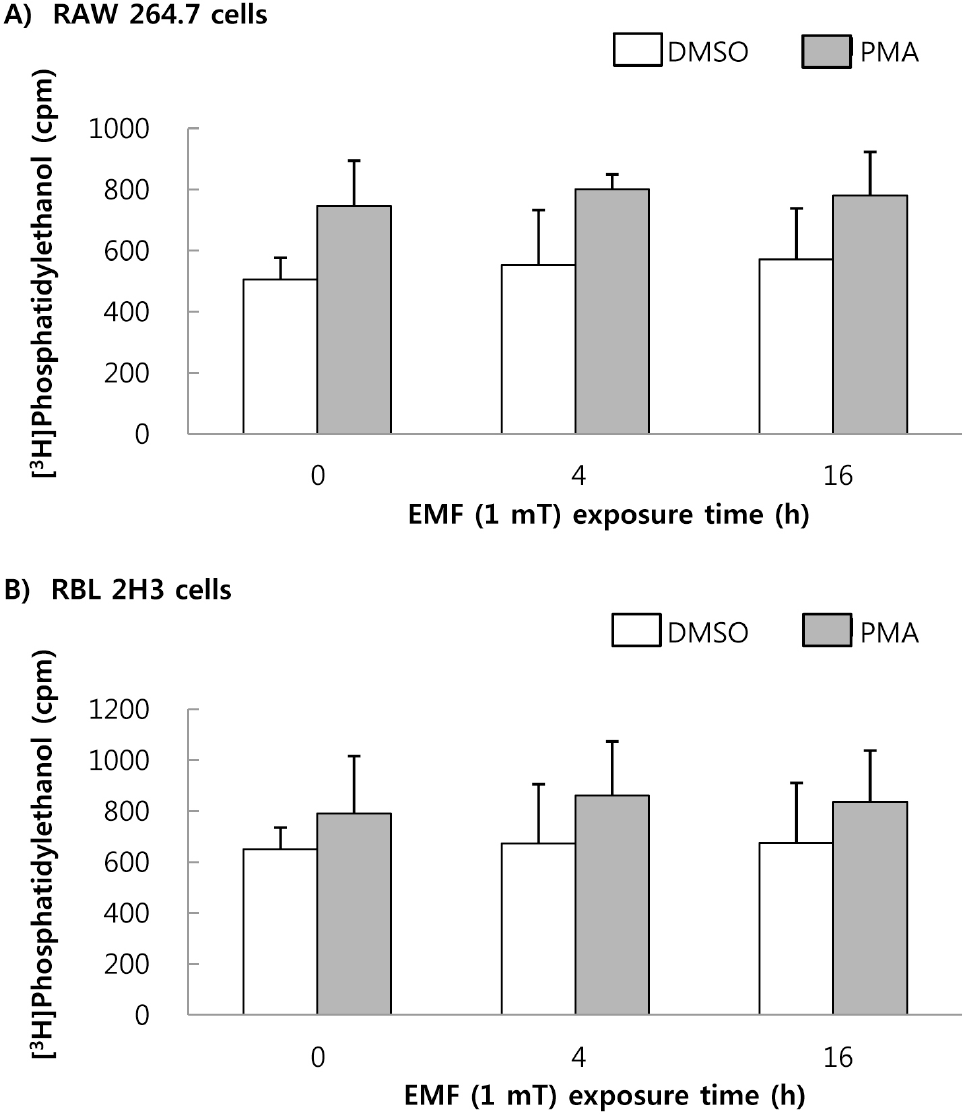 | Fig. 7.Effect of EMF on PLD activity. RAW 264.7 cells (A) and RBL 2H3 cells (B) were exposed to EMF (60 Hz, 1 mT) for 4 or 16 hand were labeled with [3H]oleic acid, for 3 h. The radioactivity of [3H]phosphatidylethanol produced by PLD was measured in the presence or absence of 1μM PMA. Results indicate mean±S.D. from four separate experiment. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download