Abstract
Mast cells are activated by specific allergens and also by various nonspecific stimuli, which might induce physical urticaria. This study investigated the functional expression of temperature sensitive transient receptor potential vanilloid (TRPV) subfamily in the human mast cell line (HMC-1) using whole-cell patch clamp techniques. The temperature of perfusate was raised from room temperature (RT, 23∼25°C) to a moderately high temperature (MHT, 37∼39°C) to activate TRPV3/4, a high temperature (HT, 44∼46°C) to activate TRPV1, or a very high temperature (VHT, 53∼55°C) to activate TRPV2. The membrane conductance of HMC-1 was increased by MHT and HT in about 50% (21 of 40) of the tested cells, and the I/V curves showed weak outward rectification. VHT-induced current was 10-fold larger than those induced by MHT and HT. The application of the TRPV4 activator 4α-phorbol 12,13-didecanoate (4α PDD, 1 μM) induced weakly outward rectifying currents similar to those induced by MHT. However, the TRPV3 agonist camphor or TRPV1 agonist capsaicin had no effect. RT-PCR analysis of HMC-1 demonstrated the expression of TRPV4 as well as potent expression of TRPV2. The [Ca2+]c of HMC-1 cells was also increased by MHT or by 4α PDD. In summary, our present study indicates that HMC-1 cells express Ca2+-permeable TRPV4 channels in addition to the previously reported expression of TRPV2 with a higher threshold of activating temperature.
Go to : 
References
1. Bradding P, Holgate ST. Immunopathology and human mast cell cytokines. Crit Rev Oncol Hematol. 1999; 31:119–133.

2. Yong LC. The mast cell: origin, morphology, distribution, and function. Exp Toxicol Pathol. 1997; 49:409–424.

3. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cell as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005; 23:749–786.
4. Bradding P, Walls AF, Church MK. Mast cells and basophils: their role in initiating and maintaining inflammatory responses. Holgate ST, editor. ed.Immunopharmacology of the Respiratory System. 1st ed.London: Academic Press;1995. p. 53–84.
7. Daman L, Lieberman P, Ganier M, Hashimoto K. Localized heat urticaria. J Allergy Clin Immunol. 1978; 61:273–278.

9. Caulfield JP, Lewis RA, Hein A, Austen KF. Secretion in dissociated human pulmonary mast cells. Evidence for solubilization of granule contents before discharge. J Cell Biol. 1980; 85:299–312.

10. Bradding P. Mast cell ion channels. Saito H, Okayama Y, editors. Mast cells in allergic diseases. Chem Immunol Allergy. Basel: Karger.;2005. 87:p. 163–178.

11. Fasolato C, Hoth M, Matthews G, Penner R. Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proc Natl Acad Sci USA. 1993; 90:3068–3072.
12. Hoth M, Panner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992; 355:353–356.

13. Bradding P, Okayama Y, Kambe N, Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukoc Biol. 2003; 73:614–620.

15. Alexander SP, Mathie A, Peters JA. Guide to receptors and channels, 1st edition. Br J Pharmacol. 2004; 141 Suppl. 1:S1–126.
16. Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels R. Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem. 2000; 275:3963–3969.
17. Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol. 2000; 527:239–248.
18. Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem. 2001; 276:1020–1025.
19. Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med. 2004; 200:137–147.

20. Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002; 23:183–191.

21. Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002; 42:873–888.

22. Benham CD, Martin JG, John BD. TRPV channels as temperature sensors. Cell Calcium. 2003; 33:479–487.

23. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824.

25. Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999; 398:436–441.

26. Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntrye P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. science. 2002; 296:2046–2049.

27. Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002; 418:186–190.

28. Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002; 418:181–186.

29. Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002; 22:6408–6414.

30. Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002; 277:47044–47051.

31. Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003; 39:497–511.

32. Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJL, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000; 88:205–215.

33. Park SJ, Choi WW, Kwon OS, Chung JH, Eun HC, Earm YE, Kim SJ. Acidic pH-activated Cl- current and intracellular Ca2+ response in human keratinocytes. Korean J Physiol Pharmacol. 2008; 12:177–183.
34. Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988; 12:345–355.

35. Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003; 424:434–438.

36. Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernández-Fernández JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Cell Biol. 2008; 181:143–155.
Go to : 
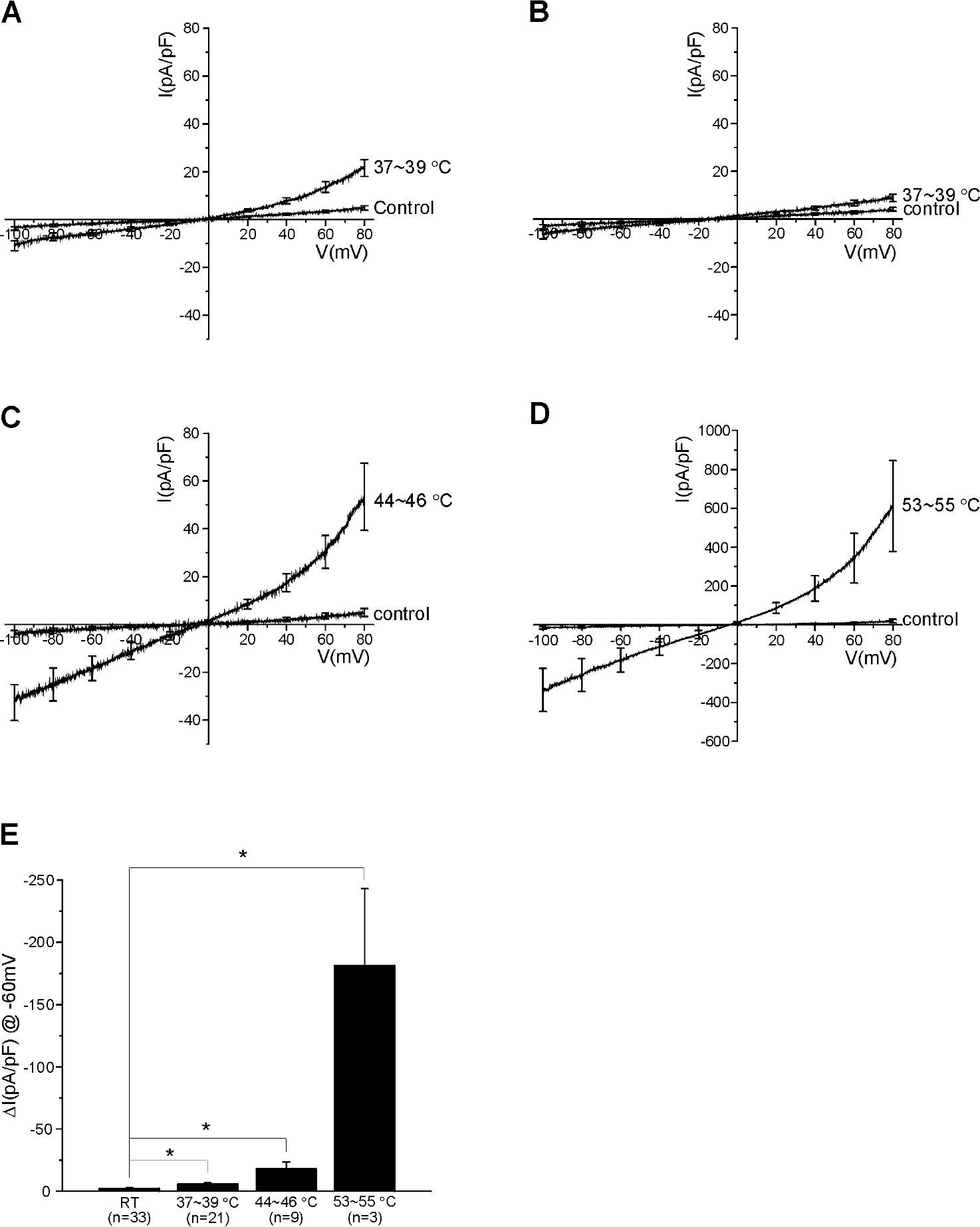 | Fig. 1.Heat-evoked currents observed at various ranges of temperature in HMC-1 cells. Current-voltage relations (I-V curves) obtained using the ramp-like pulses from –100 to 80 mV at room temperature (23∼25°C) and at different levels of increased temperature. The average values of normalized currents (pA/pF) are plotted against voltage. The responses to (MHT, 37∼39°C) are divided into two groups; relatively large increase weakly outward rectifying I/V curves (n=21, A) and small increase with linear I/V curves (n=19, B). (C) Summary of the responses to high temperature stimuli (HT, 44∼46°C, n=9) showing weakly outward rectifying I/V curves. (D) Remarkable increase of membrane conductance by very high temperature (VHT, 53∼55°C, n=3). (E) Summaries of the average densities of heat-activated current for the control, MHT, HT and VHT at –60. The data acquired from (B) were excluded in this statistics. ∗p<0.05; control versus MHT, HT and VHT, respectively. |
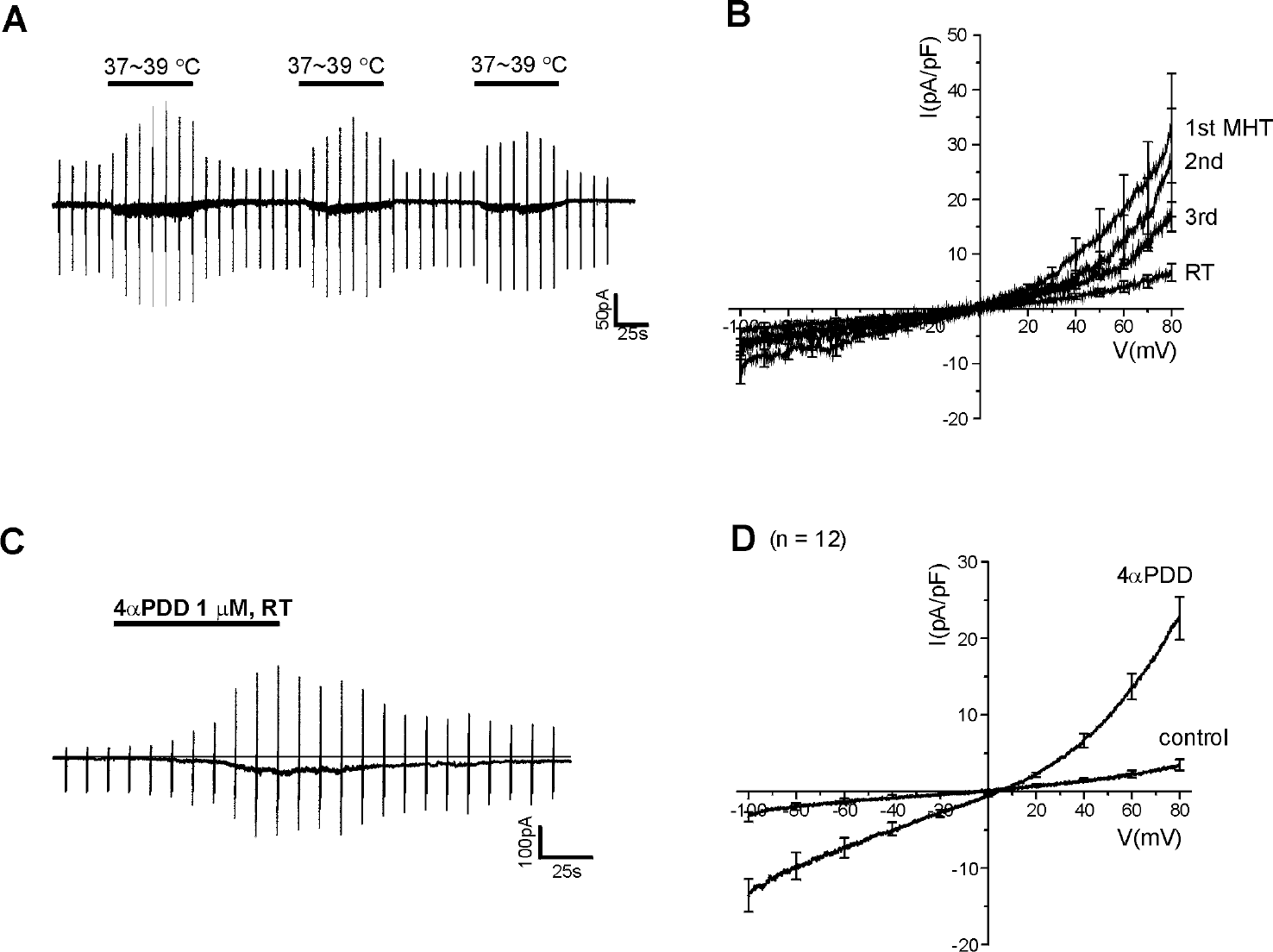 | Fig. 2.Desensitization of MHT-induced current and activation by 4aPDD in HMC-1 cells. (A) An exemplary current trace showing of repetitive application of MHT. Vertical lines reflect current responses to the repetitive ramp-like pulses from –100 to 80 mV. (B) Summary of I/V curves obtained by the ramp pulses, showing desensitization by repeated MHT stimuli (1st, 2nd, and 3rd, n=6) from room temperature (RT). The whole-cell current responded to repeated application of heat stimuli was measured using the perforated-patch recording mode. (C) Representative current trace showing the response to 1μM 4αPDD. (D) Summary of I-V curves obtained using the ramp-like pulse from –100 to 80 mV at room temperature, showing a weakly outward rectifying current induced by 1μM 4αPDD (n=12). |
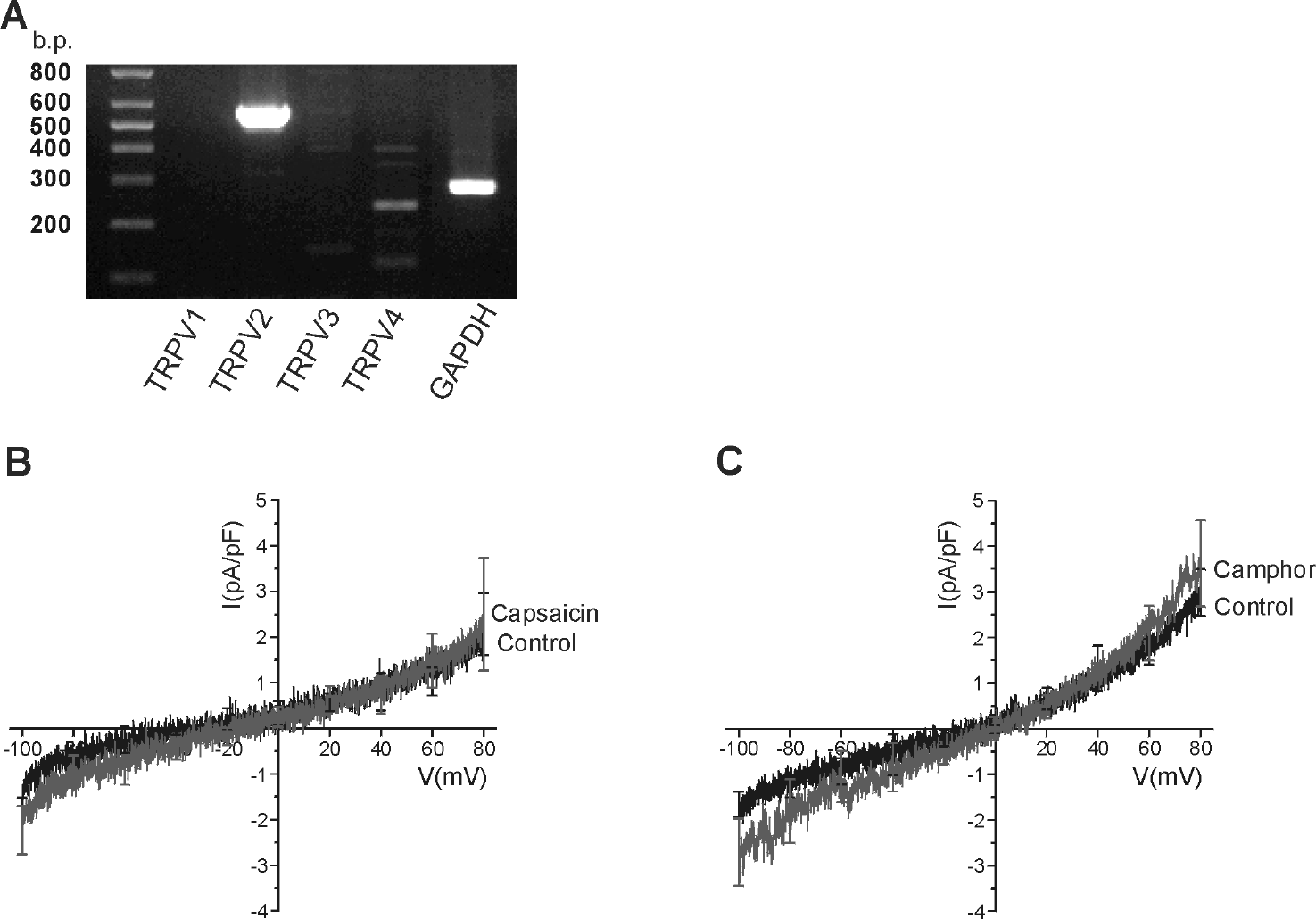 | Fig. 3.Expression of TRPV2 and TRPV4 in HMC-1 cells. (A) RT-PCR analysis for TRPV1–4 in HMC-1 cells. Positive signals corresponding to the expected sizes of TRPV2 (573 b.p.) and TRPV4 (244 b.p.) were detected while not for TRPV1 (268 b.p.) and TRPV3 (772 b.p.). GAPDH was used as the control (rightmost lane). (B) and (C) Summary of the I-V curves obtained during the applications of 2μM capsaicin (n=4) or 2 mM camphor (n=3), respectively. |
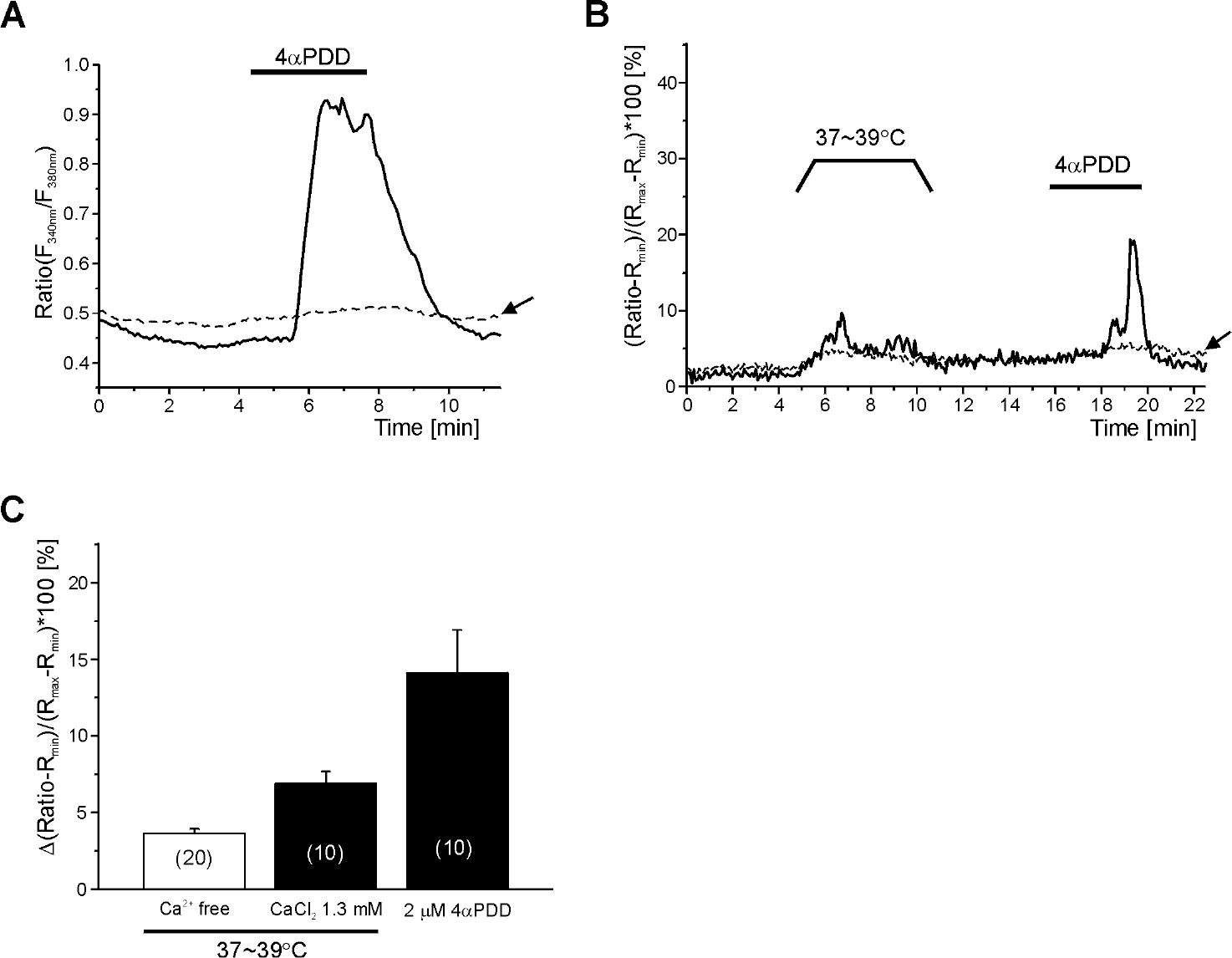 | Fig. 4.Increase of intracellular Ca2+ concentration by heat and 4αPDD in HMC-1 cells. (A) Representative traces of F340/F380 showing the effect of 2μM 4αPDD at room temperature. The gray trace indicated by arrow is a representative negative response to 4αPDD. (B) Representative traces of normalized F340/F380 (see Methods), showing the sequential increases of [Ca2+]c by MHT and 2μM 4αPDD. The gray trace indicated by arrow is a representative negative response to 4αPDD with slight increase by MHT. (C) Summary of [Ca2+]c increases caused by 2μM 4αPDD and MHT in presence (left bar) and absence (middle bar) of 1.3 mM CaCl2. Number of tested cells are indicated in each bar. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download