Abstract
To understand the function of edges in perception of moving objects, we defined four questions to answer. Is the focus point in visual motion detection of a moving object: (1) the body or the edge of the object, (2) the leading edge or trailing edge of the object, (3) different in scotopic, mesopic and photopic luminance levels, or (4) different for colored objects? We measured the Optomotor Response (OMR) and Edge Triggering Response (ETR) of goldfish. We used a square and sine wave patterns with black and red stripes and a square wave pattern with black and grey stripes to generate OMR's and ETR's in the goldfish. When we used black and red stripes, the black leading edges stimulated an ETR under scotopic conditions, red leading edges stimulated an ETR under photopic conditions, and both black and red leading edges stimulated an ETR under mesopic luminance levels. For black and gray stripes, only black leading edges stimulated an ETR in all three light illumination levels. We observed less OMR and ETR results using the sine wave pattern compared to using the square wave pattern. From these results, we deduced that the goldfish tend to prefer tracking the leading edge of the pattern. The goldfish can also detect the color of the moving pattern under photopic luminance conditions. We decided that ETR is an intriguing factor in OMR, and is suitable as a method of behavioral measurement in visual system research.
Go to : 
References
1. Field DJ, Tolhurst DJ. The structure and symmetry of simple-cell receptive-field profiles in the cat's visual cortex. Proc R Soc Lond B Biol Sci. 1986; 228:379–400.
2. Hesse GS, Georgeson MA. Edge and bars: Where do people see features in 1-D images? Vision Res. 2005; 45:507–525.
3. Kern R, Egelhaaf M, Srinivasan MV. Edge detection by landing honeybees: Behavioural analysis and model simulations of the underlying mechanism. Vision Res. 1997; 37:2103–2117.

4. Kulikowski JJ, Abadi R, King-Smith PE. Orientational selectivity of grating and line detectors in human vision. Vision Res. 1973; 13:1479–1486.

5. Marr D, Hildreth E. Theory of edge detection. Proc R Soc Lond B Biol Sci. 1980; 207:187–217.
6. Morrone MC, Burr DC. Feature detection in human vision: a phase-dependent energy model. Proc R Soc Lond B Biol Sci. 1988; 235:221–245.
8. Tolhurst DJ. On the possible existence of edge detector neurones in the human visual system. Vision Res. 1972; 12:797–804.

9. Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966; 187:517–552.

10. Goodwin AW, Henry GH, Bishop PO. Direction selectivity of simple striate cells: properties and mechanism. J Neurophysiol. 1975; 38:1500–1523.

11. Dreher B, Sanderson KJ. Receptive field analysis: Responses to moving visual contours by single lateral geniculate neurones in the cat. J Physiol. 1973; 234:95–118.

12. Cronly-Dillon JR, Muntz WRA. The spectral sensitivity of the goldfish and the clawed toad tadpole under photopic conditions. J Exp Biol. 1965; 42:481–493.

13. Schaerer S, Neumeyer C. Motion detection in goldfish investigated with the optomotor response is “color blind”. Vision Res. 1996; 36:4025–4034.

14. Hood DC, Finkelstein MA. Sensitivity to light. Boff KR, Kaufman L, Thomas JP, editors. Handbook of Perception and Human Performance. New York: Wiley;1986. p. 5–1–5–66.
15. Lee SY, Jung CS. Intraocular injection of muscimol induces illusory motion reversal in goldfish. Korean J Physiol Pharmacol. 2009; 13:469–473.

16. Anstis S, Hutahajan P, Cavanagh P. Optomotor test for wavelength sensitivity in Guppyfish (Poecilia reticylata). Vision Res. 1998; 38:45–53.
17. Faber DS, Fetcho JR, Korn H. Neuronal networks underlying the escape response in goldfish. General implications for motor control. Ann NY Acad Sci. 1989; 563:11–33.
Go to : 
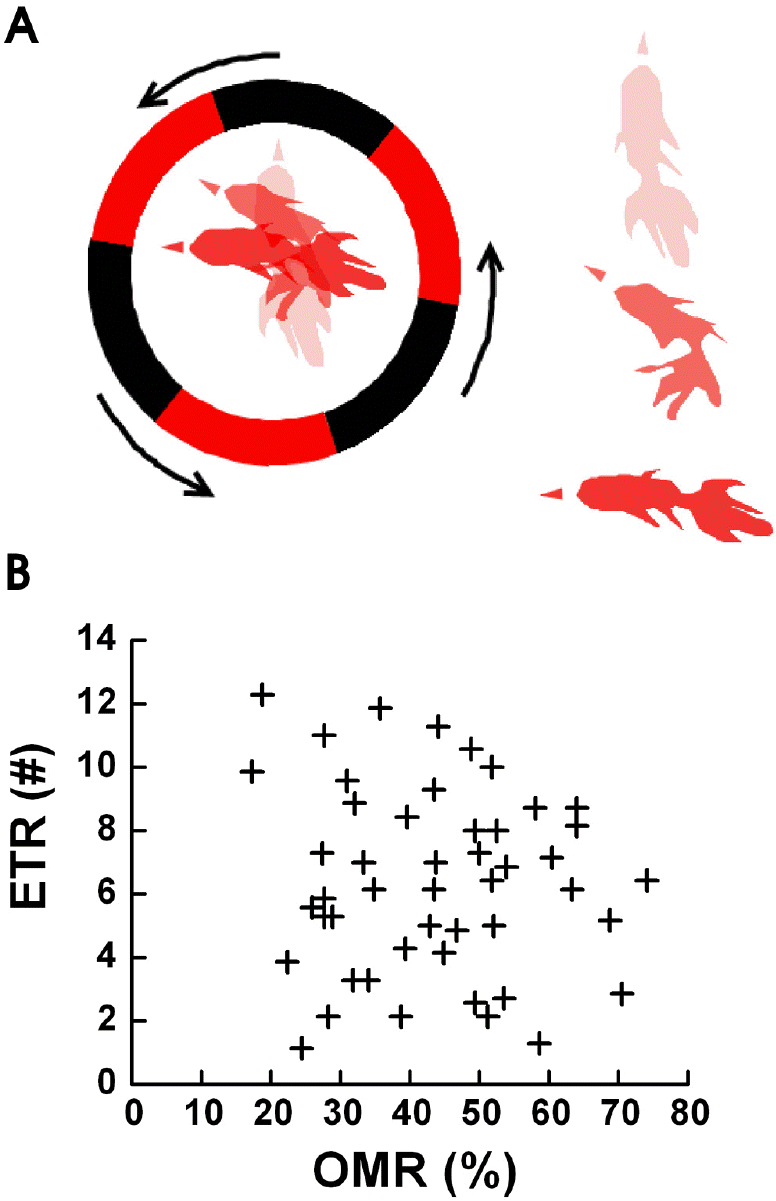 | Fig. 1.The Definition of ETR (A) and ETR versus OMR (B). (A) We defined one ETR as when the head of the goldfish in the fish bowl starts to turn as the fish tracks a single edge of the black/red square wave pattern and turns at least 90 degrees (left side of Fig. 1A in circle) from the initial direction of head. The initial position of the goldfish is indicated by the lightest drawing, the next position is a darker drawing, and the last position is the darkest drawing (the point when the goldfish turned 90 degrees). On the right side of fig. 1a, the overlapped three stages of one goldfish ETR are shown separately from top to bottom. (B) A correlation graph between OMR and ETR from experiments with 50 goldfish. Both OMR and ETR were in a wide range from 1 to 12 or 20% to 75% and had mean values of OMR=43.47±4.34% and ETR=6.38±2.91. |
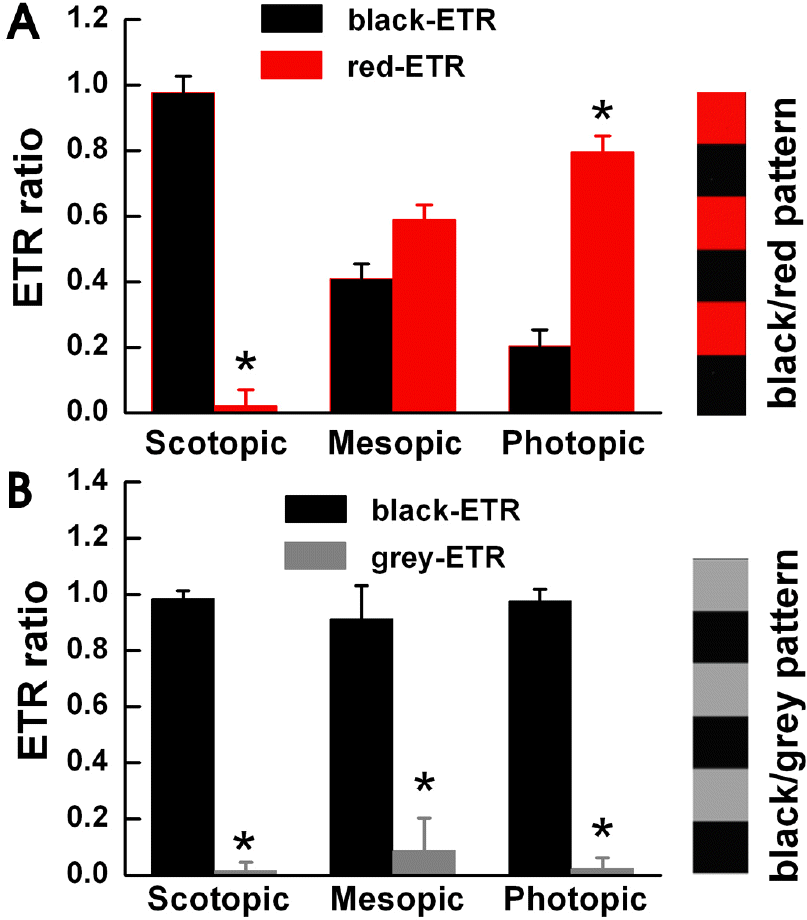 | Fig. 2.The goldfish detects movement of color under photopic luminance conditions. We allowed the goldfish to adapt to the three light illumination conditions. We measured the ETR ratio using a square wave black/red pattern (n=5) in Fig. 2A and a black/grey pattern in Fig. 2B. (A) We observed mostly black-ETR's under scotopic conditions, more red-ETR's than black-ETR's under photopic conditions, and almost equal amounts under mesopic conditions. Each specimen displayed different results when stimulated with a black/grey pattern. (B) We used black/grey pattern stripes with identical contrast levels to the black/red pattern stripes used in Fig. 2A. Under all light conditions a black-ETR was the most frequent result. Before the experiment, we allowed each goldfish to adapt to its light level for about 20 minutes. Under the three light illumination levels, we measured similar contrast results of 0.4 (scotopic), 0.42 (mesopic), and 0.43 (photopic). Each bar represents mean±S.E.M. ∗p<0.05 compared with black-ETR. |
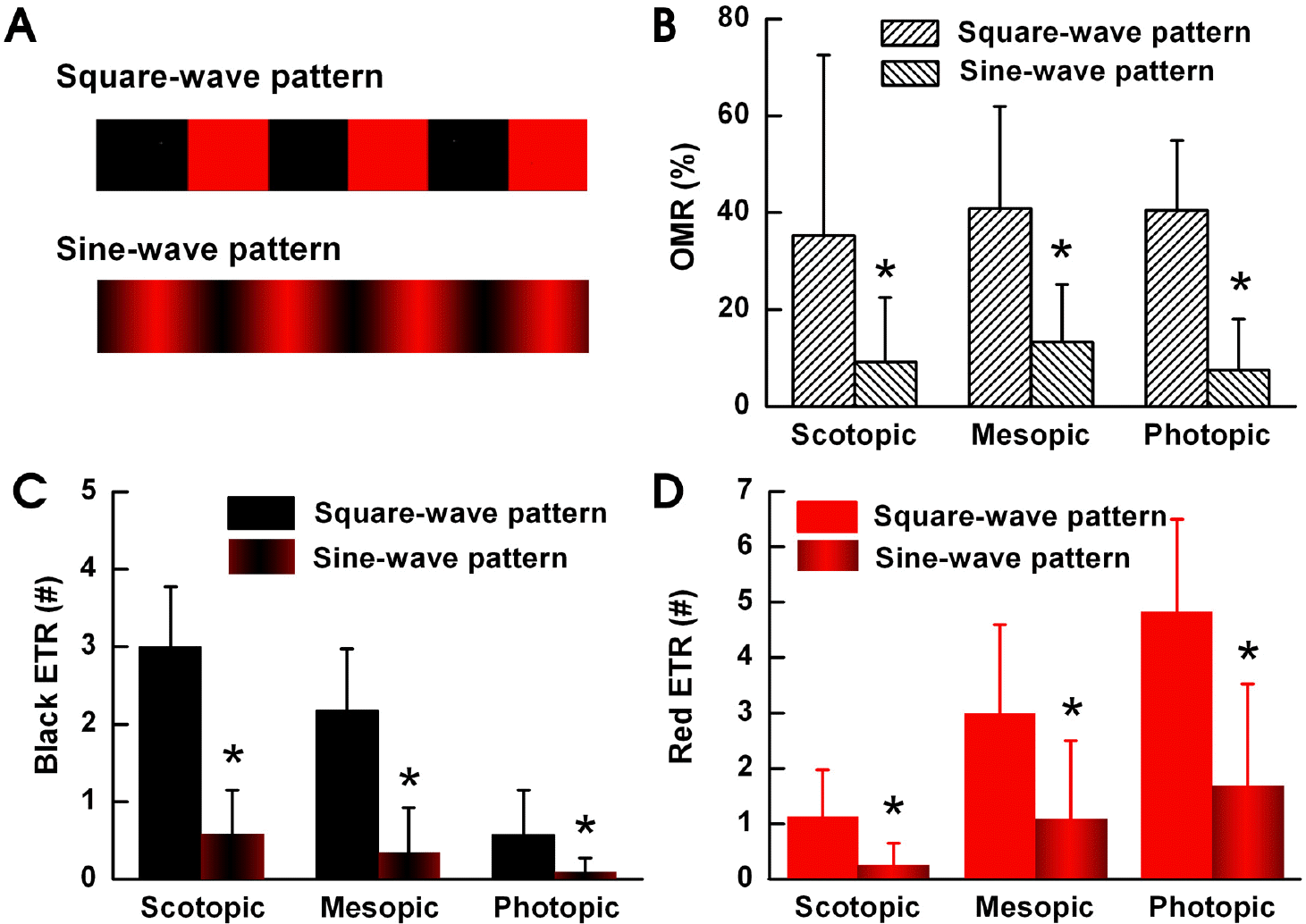 | Fig. 3.OMR occurs when an edge is perceived. We measured the OMR and ETR responses induced by the square and sine wave patterns as shown on (A) under each light illumination condition (n=8). (B) Under all three light illumination conditions, the OMR results decreased significantly when we changed to using the sine wave pattern. (C) Under all three light illumination conditions the black-ETR results from using the square wave pattern were significantly higher than when we used the sine wave pattern. The black-ETR from the square wave or sine wave pattern was the greatest under scotopic conditions and decreased as the surroundings brightened. (D) The red-ETR from the square wave or sine wave pattern was the lowest under scotopic conditions and increased as the surroundings brightened. Under all three light illumination conditions, the red-ETR results decreased significantly when we used the sine wave pattern. ∗p<0.05 compared with square-wave pattern. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download