Abstract
3-Deazaadenosine (DZA), a potent inhibitor of S-adenosylhomocysteine hydrolase, was previously proposed to induce intrinsic apoptosis in human leukemic cells. In the present study, we analyzed the mechanism underlying the DZA-induced intrinsic apoptotic pathway. DZA activated typical caspase-dependent apoptosis in HL-60 cells, as demonstrated by an accumulation of hypo-diploidic cells, the processing of multiple procaspases and an inhibitory effect of z-VAD-Fmk on this cell death. During DZA-induced apoptosis, cytochrome c (cyt c) was released into the cytosol. This was neither prevented by z-VAD-Fmk and nor was it associated with the dissipation of mitochondrial membrane potential (ΔΨm). Prior to the release of cyt c, BAX was translocated from the cytosol to mitochondria and underwent oligomerization. Finally, the overexpression of BCL-XL protected HL-60 cells from apoptosis by blocking both the cyt c release and BAX oligomerization. Collectively, these findings suggest that DZA may activate intrinsic apoptosis by stimulating BAX activation and thereby the release of cyt c.
Go to : 
References
1. Chiang PK, Richards HH, Cantoni GL. S-Adenosyl-L-homocysteine hydrolase: analogues of S-adenosyl-L-homocysteine as potential inhibitors. Mol Pharmacol. 1977; 13:939–947.
2. Aksamit RR, Falk W, Cantoni GL. Inhibition of chemotaxis by S-3-deazaadenosylhomocysteine in a mouse macrophage cell line. J Biol Chem. 1982; 257:621–625.

3. Chiang PK, Cantoni GL. Perturbation of biochemical transmethylations by 3-deazaadenosine in vivo. Biochem Pharmacol. 1979; 28:1897–1902.

4. Mayers DL, Mikovits JA, Joshi B, Hewlett IK, Estrada JS, Wolfe AD, Garcia GE, Doctor BP, Burke DS, Gordon RK, Lane JR, Chiang PK. Anti-human immunodeficiency virus 1 (HIV-1) activities of 3-deazaadenosine analogs: increased potency against-3′-azido-3′-deoxythymidine-resistant HIV-1 strains. Proc Natl Acad Sci USA. 1995; 92:215–219.
5. Krenitsky TA, Rideout JL, Chao EY, Koszalka GW, Gurney F, Crouch RC, Cohn NK, Wolberg G, Vinegar R. Imidazo[4,5-c] pyridines (3-deazapurines) and their nucleosides as immunosuppressive and antiinflammatory agents. J Med Chem. 1986; 29:138–143.
6. Chiang PK, Burbelo PD, Brugh SA, Gordon RK, Fukuda K, Yamada Y. Activation of collagen IV gene expression in F9 teratocarcinoma cells by 3-deazaadenosine analogs. Indirect inhibitors of methylation. J Biol Chem. 1992; 267:4988–4991.

7. Jurgensen CH, Huber BE, Zimmerman TP, Wolberg G. 3-deazaadenosine inhibits leukocyte adhesion and ICAM-1 biosynthesis in tumor necrosis factor-stimulated human endothelial cells. J Immunol. 1990; 144:653–661.
8. Jeong SY, Lee JH, Kim HS, Hong SH, Cheong CH, Kim IK. 3-Deazaadenosine analogues inhibit the production of tumour necrosis factor-α in RAW264.7 cells stimulated with lipopolysaccharide. Immunology. 1996; 89:558–562.
9. Jeong SY, Ahn SG, Lee JH, Kim HS, Kim JW, Rhim H, Jeong SW, Kim IK. 3-deazaadenosine, a S-adenosylhomocysteine hydrolase inhibitor, has dual effects on NF-kappaB regulation. Inhibition of NF-kappaB transcriptional activity and promotion of IκBα degradation. J Biol Chem. 1999; 274:18981–18988.
10. Endresen PC, Eide TJ, Aarbakke J. Cell death initiated by 3-deazaadenosine in HL-60 cells is apoptosis and is partially inhibited by homocysteine. Biochem Pharmacol. 1993; 46:1893–1901.

11. Kim HS, Jeong SY, Lee JH, Kim BE, Kim JW, Jeong SW, Kim IK. Induction of apoptosis in human leukemia cells by 3-deazaadenosine is mediated by caspase-3-like activity. Exp Mol Med. 2000; 32:197–203.

12. Kim IK, Li CC, Young HA, Lee JH, Kim HS, Pardhasaradhi K, Garcia GE, Chiang PK. Apoptosis of L1210 Leukemia Cells Induced by 3-Deazaadenosine Analogs: Differential Expression of c-myc, NF-κB and Molecular Events. J Biomed Sci. 1997; 4:83–90.
13. Chae YJ, Kim HS, Rhim H, Kim BE, Jeong SW, Kim IK. Activation of caspase-8 in 3-deazaadenosine-induced apoptosis of U-937 cells occurs downstream of caspase-3 and caspase-9 without Fas receptor-ligand interaction. Exp Mol Med. 2001; 33:284–292.

14. Byun HS, Won M, Park KA, Kim YR, Choi BL, Lee H, Hong JH, Piao L, Park J, Kim JM, Kweon GR, Kang SH, Han J, Hur GM. Prevention of TNF-induced necrotic cell death by rottlerin through a Nox1 NADPH oxidase. Exp Mol Med. 2008; 40:186–195.

15. Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH. Narigin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol. 2009; 13:281–285.
16. Lee SY, Ahn JH, Ko KW, Kim J, Jeong SW, Kim IK, Kim J, Kim HS. Prostaglandin A2 activates intrinsic apoptotic pathway by direct interaction with mitochondria in HL-60 cells. Prostaglandins Other Lipid Mediat. 2010; 91:30–37.

17. Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005; 102:17975–17980.

18. Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, Andreeff M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010; 120:142–156.

19. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004; 305:626–629.

20. Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010; 11:621–632.

21. van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006; 16:203–213.

22. Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0)F(1)-ATPase. J Biol Chem. 2001; 276:6453–6462.
23. Li TW, Zhang Q, Oh P, Xia M, Chen H, Bemanian S, Lastra N, Circ M, Moyer MP, Mato JM, Aw TY, Lu SC. S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol Pharmacol. 2009; 76:192–200.

24. Huang RF, Huang SM, Lin BS, Wei JS, Liu TZ. Homocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL-60 cells. Life Sci. 2001; 68:2799–2811.

25. Cho YW, Jung HJ, Kim YK, Woo JS. A1 receptor-mediated protection against amyloid beta-induced injury in human neuroglioma cells. Korean J Physiol Pharmacol. 2007; 11:37–43.
26. Zhenchuk A, Lotfi K, Juliusson G, Albertioni F. Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharmacol. 2009; 78:1351–1359.

27. Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, Shortnacy AT, Montgomery JA, Secrist JA 3rd, Bennett LL Jr. Incorporation of the carbocyclic analog of 2′-deoxyguanosine into the DNA of herpes simplex virus and of HEp-2 cells infected with herpes simplex virus. Cancer Res. 1991; 51:2386–2394.
28. Genini D, Budihardjo I, Plunkett W, Wang X, Carrera CJ, Cottam HB, Carson DA, Leoni LM. Nucleotide requirements for the in vitro activation of the apoptosis protein-activating factor-1-mediated caspase pathway. J Biol Chem. 2000; 275:29–34.

29. Prus KL, Wolberg G, Keller PM, Fyfe JA, Stopford CR, Zimmerman TP. 3-Deazaadenosine 5′-triphosphate: a novel metabolite of 3-deazaadenosine in mouse leukocytes. Biochem Pharmacol. 1989; 38:509–517.

Go to : 
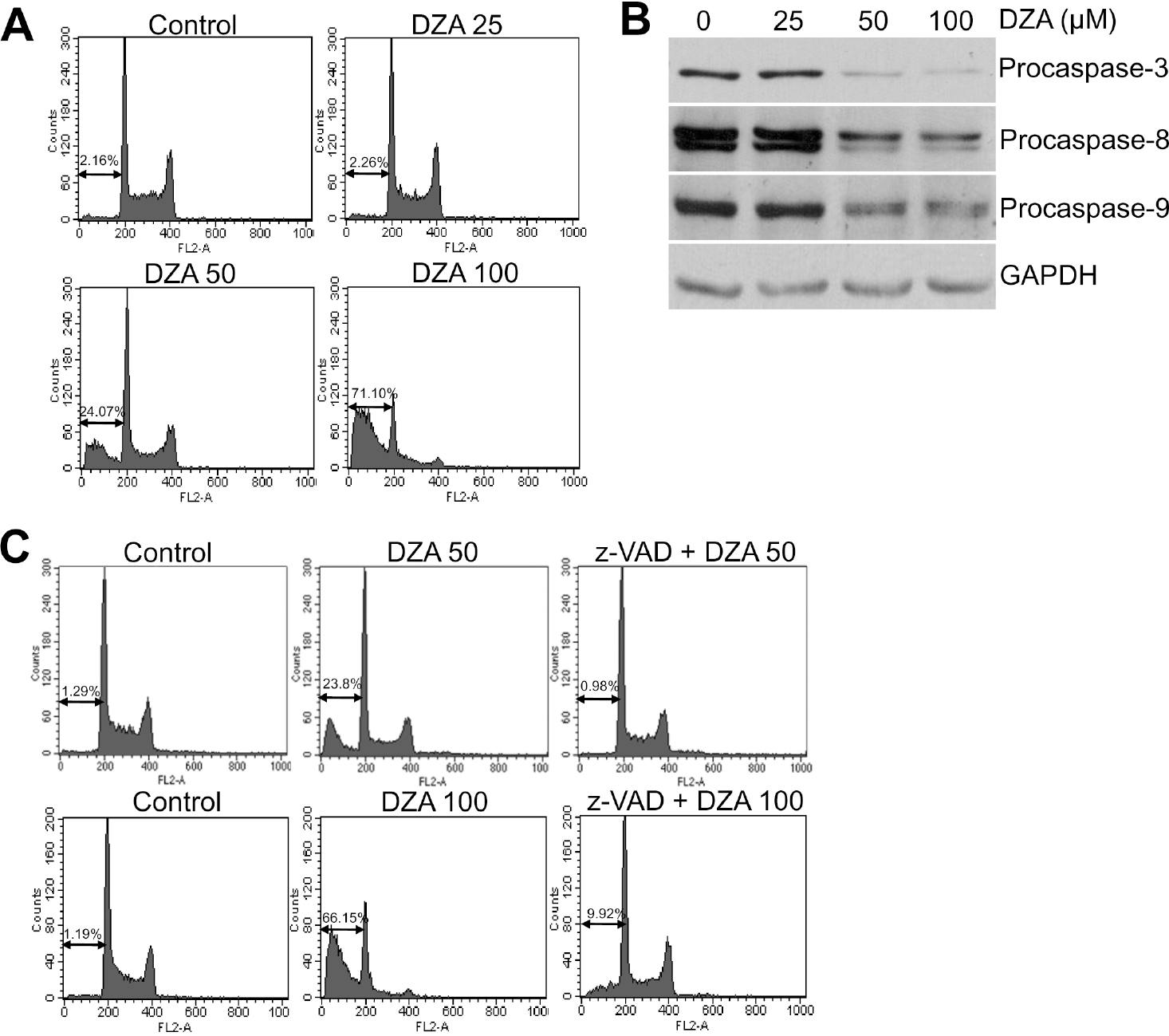 | Fig. 1.Induction of caspase-dependent apoptosis in HL-60 cells by DZA. (A) HL-60 cells treated with the indicated micromolar concentrations of DZA for 6 h were fixed, stained with PI and then analyzed by flow cytometry as described in METHODS. (B) HL-60 cells treated the same as described in (A) were subjected to immunoblot analysis against the indicated procaspases. GAPDH was used for equal loading of proteins. (C) After incubating HL-60 cells in the absence or presence of 30μM z-VAD-Fmk for 1 h, they were treated with the indicated micromolar concentrations of DZA for 6 h further. The cells then were subjected to PI staining and flow cytometric analysis. The figures are representative of three independent experiments. |
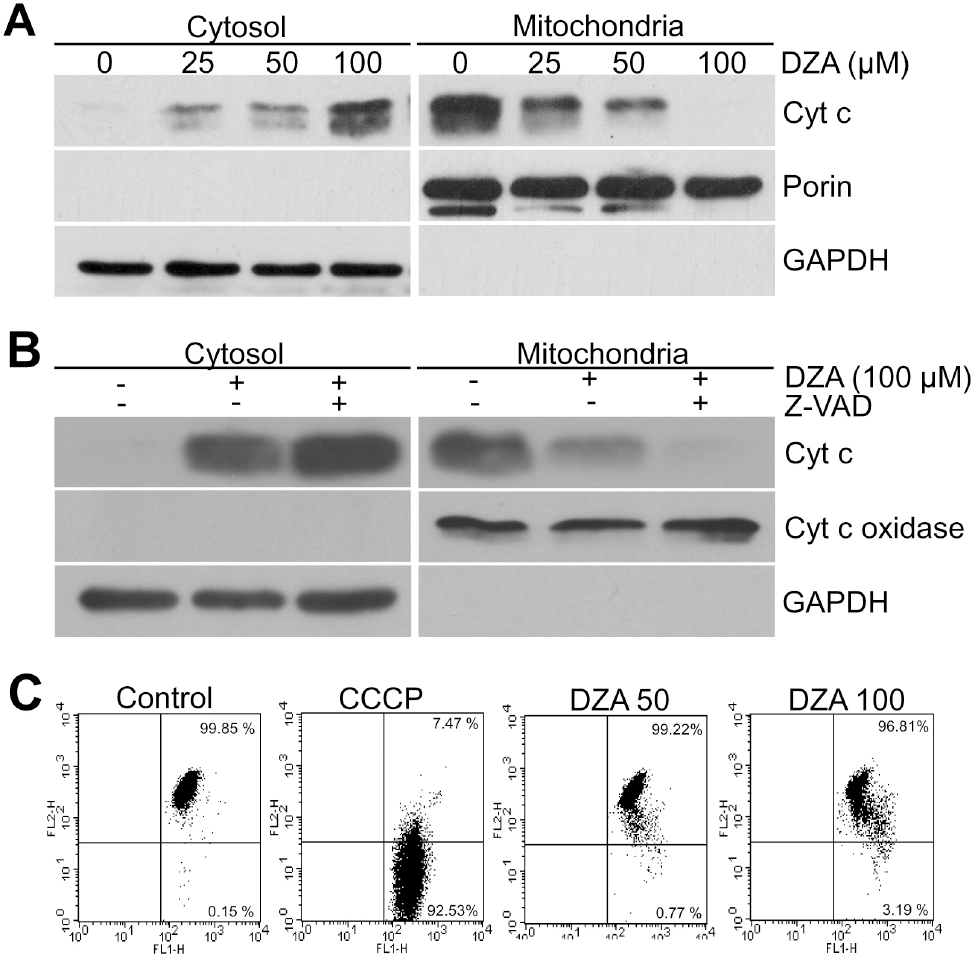 | Fig. 2.DZA-induced cyt c release in a PT- and caspase-independent manner. (A) Cytosolic and mitochondrial fractions of HL-60 cells treated with the indicated concentrations of DZA for 6 h were subjected to immunoblot analysis against cyt c. Porin and GAPDH were used for the equal loading of mitochondrial and cytosolic proteins, respectively. (B) HL-60 cells incubated in the absence or presence of 30μM z-VAD-Fmk for 1 h were treated with vehicle or 100μM DZA. Six hours later, cytosolic and mitochondrial fractions were prepared and subjected to immunoblot analysis against cyt c. Cyt c oxidase and GAPDH were used for equal loading of mitochondrial and cytosolic proteins, respectively. (C) HL-60 cells treated with vehicle, the indicated micromolar concentrations of DZA for 6 h or 50μM CCCP for 5 min were incubated with 2μM JC-1. After 15 min, cells were harvested, washed and analyzed on FACSCalibur with emission filters of FL1 and FL2 for green and red fluorescences, respectively. CCCP was used to confirm that JC-1 adequately reacted to MMP dissipation. The results are representative of three independent experiments. |
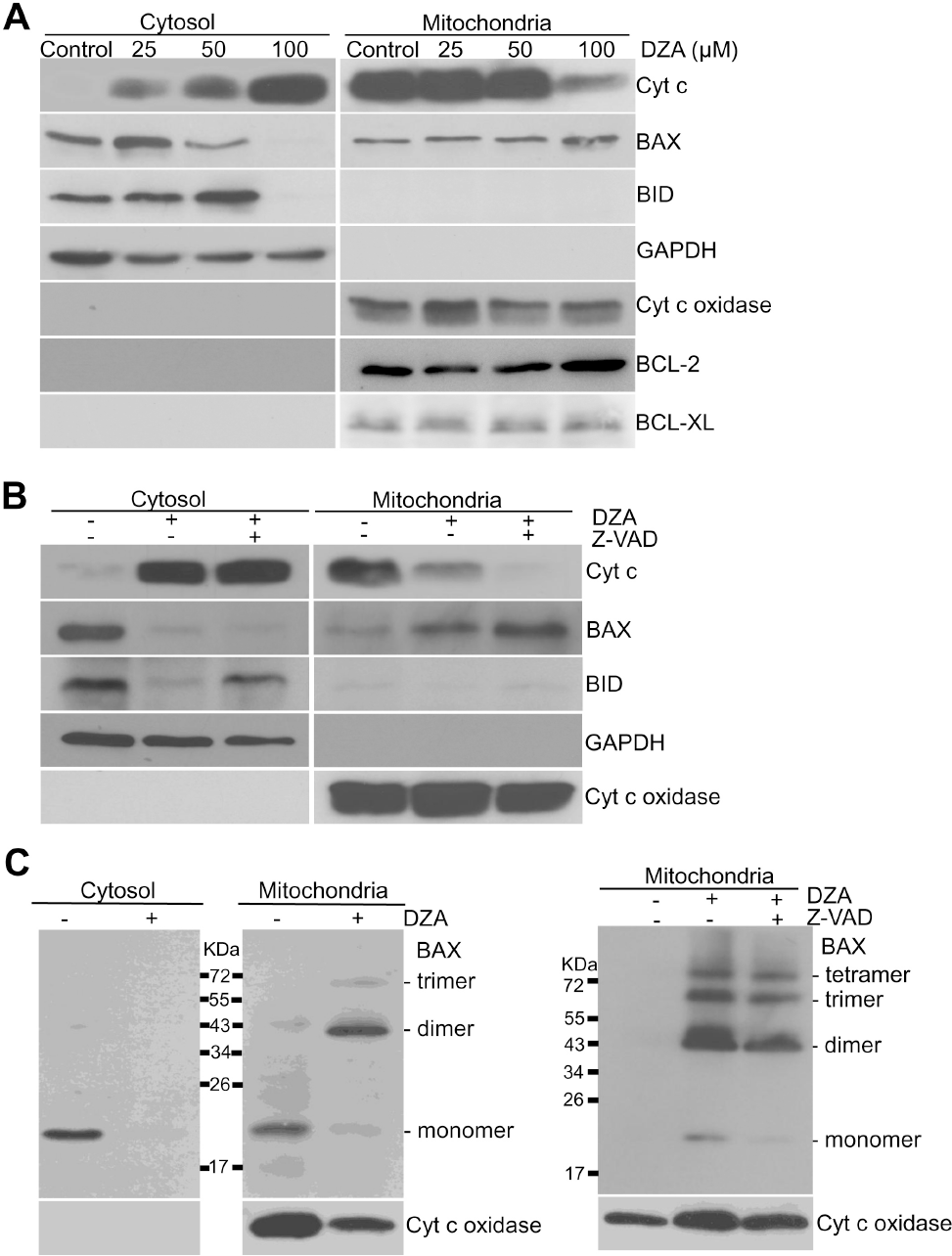 | Fig. 3.DZA-induced BAX activation in HL-60 cells. (A) Subcellular fractions of HL-60 cells treated with indicated concentrations of DZA were prepared and subjected to immunoblot analysis against the indicated proteins. GAPDH and cyt c oxidase were used for equal loading of cytosolic and mitochondrial proteins, respectively. (B) Cytosolic and mitochondrial fractions of HL-60 cells incubated with vehicle or 30μM z-VAD-Fmk for 1 h and then 100μM DZA for 6 h further were prepared and subjected to immunoblot analysis. Cyt c oxidase and GAPDH were used for equal loading of mitochondrial and cytosolic fractions, respectively. (C) Left, HL-60 cells were treated with vehicle or 100μM DZA. Right, HL-60 cells incubated in the absence or presence of 30μM z-VAD-Fmk for 1 h were treated with DZA. After 6 h, cytosolic and mitochondrial fractions were incubated in the presence of 2 mM BMH as described in METHODS. Each fraction was subjected to immunoblot analysis against BAX and cyt c oxidase. The results are representative of three independent experiments. |
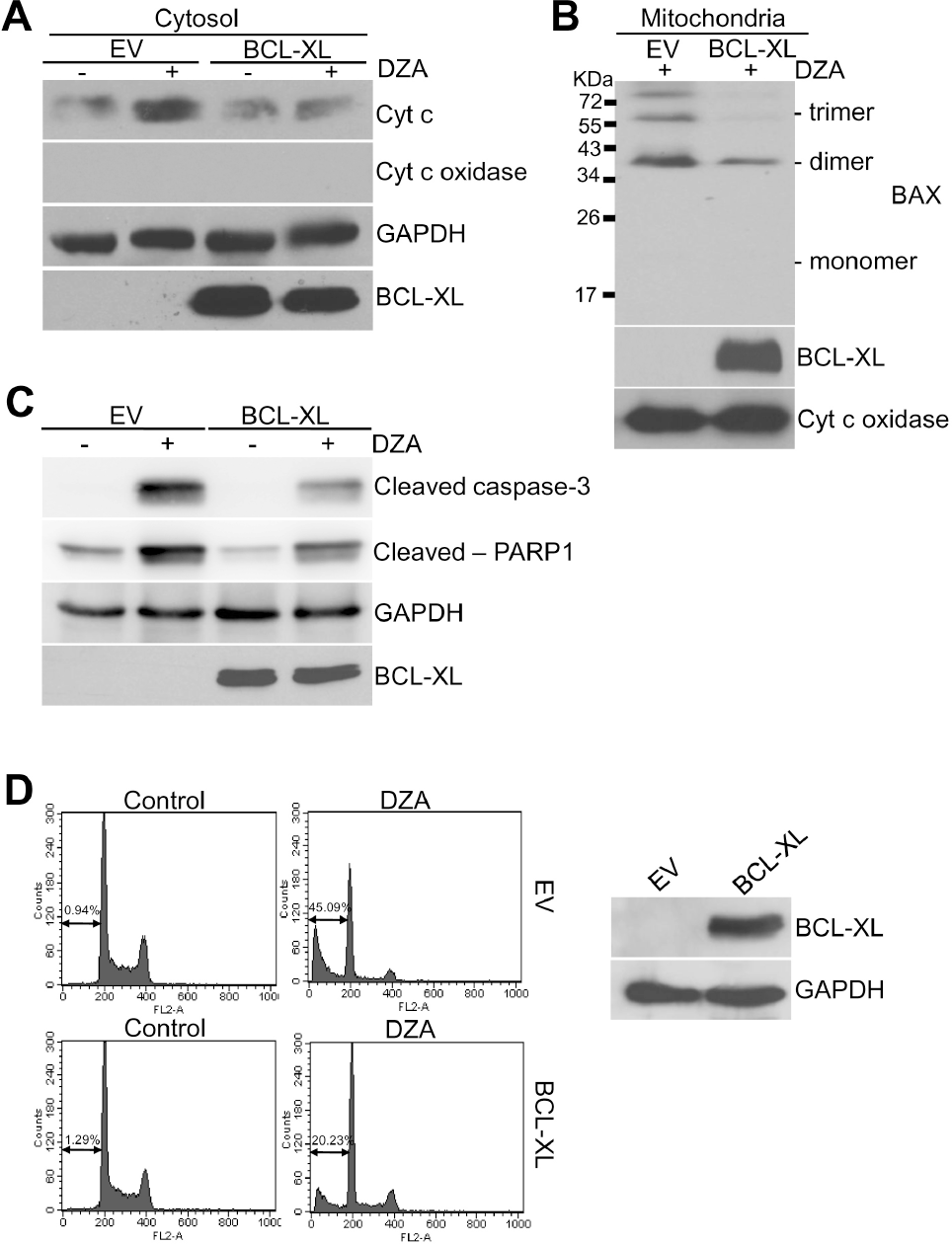 | Fig. 4.Inhibition of DZA-induced apoptosis by BCL-XL. (A) HL-60 cells transiently transfected with empty pcDNA3.1(–) vector (EV) or plasmid expressing BCL-XL (pcDNA3.1-/BCL-XL) for 48 h were treated with vehicle or 100μM DZA. After 6 h, cytosolic fractions were subjected to immunoblot analysis against cyt c. GAPDH were used for equal loading of cytosolic proteins. (B) HL-60 cells transfected the same as in experiment (A) were treated with vehicle or 100μM DZA for 6 h. Mitochondrial fractions were then treated with 2 mM BMH as described in METHODS and subjected to immunoblot analysis against BAX. Cyt c oxidase was used for equal loading of mitochondrial proteins. (C) HL-60 cells transfected as indicated were treated with vehicle or 100μM DZA for 6 h and subjected to immunoblot analysis. GAPDH were used for equal loading of cell lysates. (D) HL-60 cells transiently transfected as indicated were treated with vehicle or 100μM DZA for 6 h. Cells were then fixed and subjected to PI staining and flow cytometric analysis. The figures are representative of three independent experiments. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download