Abstract
Excessive extracellular matrix (ECM) accumulation is the main feature of chronic renal disease including diabetic nephropathy. Plasminogen activator inhibitor (PAI)-1 is known to play an important role in renal ECM accumulation in part through suppression of plasmin generation and matrix metalloproteinase (MMP) activation. The present study examined the effect of PAI-1 antisense oligodeoxynucleotide (ODN) on fibronectin upregulation and plasmin/MMP suppression in primary mesangial cells cultured under high glucose (HG) or transforming growth factor (TGF)-β1, major mediators of diabetic renal ECM accumulation. Growth arrested and synchronized rat primary mesangial cells were transfected with 1μM phosphorothioate-modified antisense or control mis-match ODN for 24 hours with cationic liposome and then stimulated with 30 mM D-glucose or 2 ng/ml TGF-β1. PAI-1 or fibronectin protein was measured by Western blot analysis. Plasmin activity was determined using a synthetic fluorometric plasmin substrate and MMP-2 activity analyzed using zymography. HG and TGF-β1 significantly increased PAI-1 and fibronectin protein expression as well as decreased plasmin and MMP-2 activity. Transient transfection of mesangial cells with PAI-1 antisense ODN, but not mis-match ODN, effectively reversed basal as well as HG- and TGF-β1-induced suppression of plasmin and MMP-2 activity. Both basal and upregulated fibronectin secretion were also inhibited by PAI-1 antisense ODN. These data confirm that PAI-1 plays an important role in ECM accumulation in diabetic mesangium through suppression of protease activity and suggest that PAI-1 antisense ODN would be an effective therapeutic strategy for prevention of renal fibrosis including diabetic nephropathy.
Go to : 
References
1. Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989; 38:1077–1081.

2. Schnaper HW. Balance between matrix synthesis and degradation: a determinant of glomerulosclerosis. Pediatr Nephrol. 1995; 9:104–111.

3. Wong AP, Cortez SL, Baricos WH. Role of plasmin and gelatinase in extracellular matrix degradation by cultured rat mesangial cells. Am J Physiol. 1992; 263:F1112–F1118.

4. Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J Am Soc Nephrol. 1999; 10:790–795.
5. Monea S, Lehti K, Keski-Oja J, Mignatti P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol. 2002; 192:160–170.

6. Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991; 88:1067–1072.

7. Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000; 58:1841–1850.

9. Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006; 17:2999–3012.

10. Ha H, Oh EY, Lee HB. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol. 2009; 5:203–211.

11. Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005; 67:1762–1771.

12. Kitching AR, Kong YZ, Huang XR, Davenport P, Edgtton KL, Carmeliet P, Holdsworth SR, Tipping PG. Plasminogen activator inhibitor-1 is a significant determinant of renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003; 14:1487–1495.

13. Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 2001; 60:587–596.

14. Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005; 67:1297–1307.

15. Collins SJ, Alexander SL, Lopez-Guisa JM, Cai X, Maruvada R, Chua SC, Zhang G, Okamura DM, Matsuo S, Eddy AA. Plasminogen activator inhibitor-1 deficiency has renal benefits but some adverse systemic consequences in diabetic mice. Nephron Exp Nephrol. 2006; 104:e23–e34.

16. Seo JY, Park J, Yu MR, Kim YS, Ha H, Lee HB. Positive feedback loop between plasminogen activator inhibitor-1 and transforming growth factor-beta1 during renal fibrosis in diabetes. Am J Nephrol. 2009; 30:481–490.

17. Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest. 2003; 112:379–388.

18. Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA. A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. J Am Soc Nephrol. 2008; 19:329–338.

19. Huang Y, Border WA, Lawrence DA, Noble NA. Mechanisms underlying the antifibrotic properties of noninhibitory PAI-1 (PAI-1R) in experimental nephritis. Am J Physiol Renal Physiol. 2009; 297:F1045–F1054.

20. Neckers L, Whitesell L. Antisense technology: biological utility and practical considerations. Am J Physiol. 1993; 265:L1–L12.

21. de Martimprey H, Vauthier C, Malvy C, Couvreur P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. Eur J Pharm Biopharm. 2009; 71:490–504.

22. Cho HK, Yang EK, Han HS, Lee WJ, Phillips MI. Effect of brain angiotensin II receptor antagonists and antisense oligonucleotide on drinking and renal renin in rats. Korean J Physiol Pharmacol. 2000; 4:137–142.
23. Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998; 54:1872–1878.
24. Jiang Z, Seo JY, Ha H, Lee EA, Kim YS, Han DC, Uh ST, Park CS, Lee HB. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun. 2003; 309:961–966.
25. Fisher EJ, McLennan SV, Yue DK, Turtle JR. High glucose reduces generation of plasmin activity by mesangial cells. Microvasc Res. 1997; 53:173–178.

26. Kanalas JJ, Hopfer U. Effect of TGF-beta 1 and TNF-alpha on the plasminogen system of rat proximal tubular epithelial cells. J Am Soc Nephrol. 1997; 8:184–192.

Go to : 
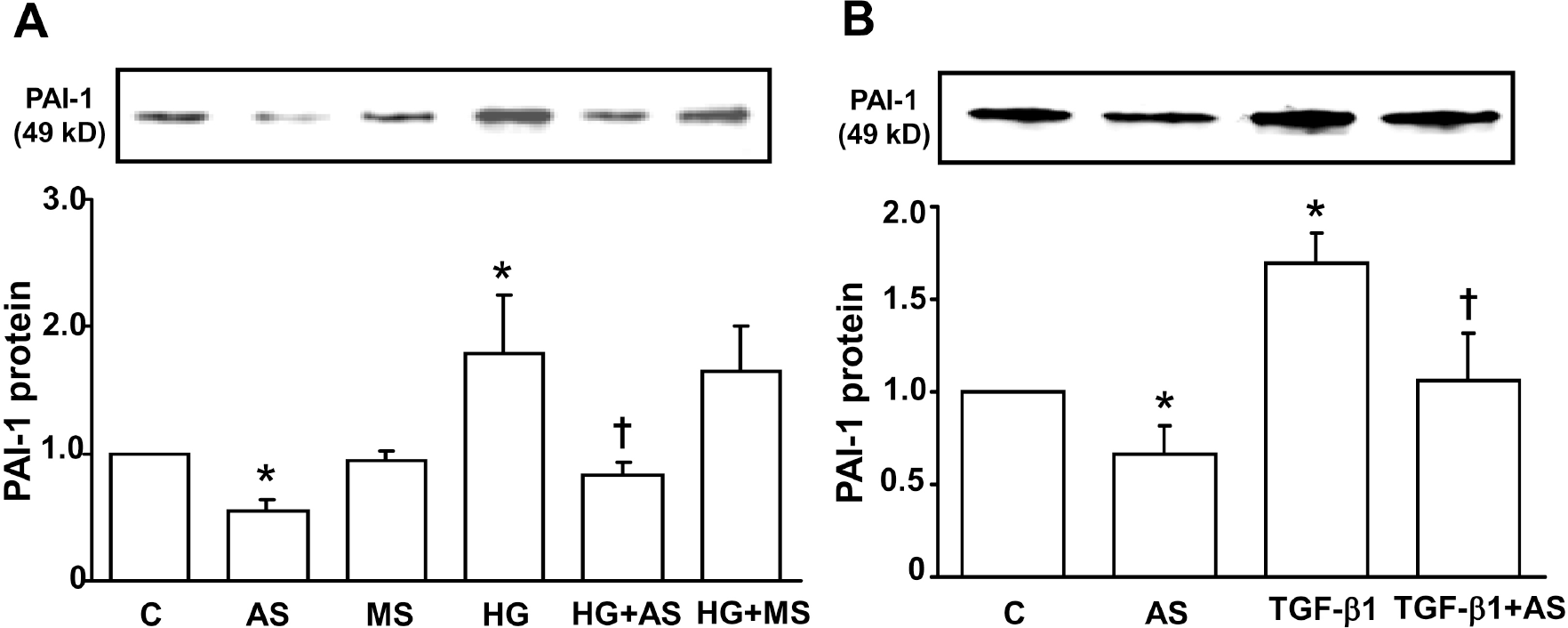 | Fig. 1.Effect of PAI-1 antisense ODN on HG- (A) and TGF-β 1- (B) induced PAI-1 protein expression in mesangial cells. Growth arrested and synchronized primary rat mesangial cells were stimulated with 30 mM high D-glucose (HG) (A) or 2 ng/ml of TGF-β 1 (B) for 48 hours after PAI-1 antisense or mis-match sense ODN transfection. PAI-1 protein was measured by Western blot analysis as described in the Methods. Data are presented as means±SE of 4 experiments. ∗p<0.05 vs control, †p<0.05 vs HG or TGF-β1, C: control without HG or TGF-β1, AS: PAI-1 antisense ODN, MS: PAI-1 mis-match sense ODN. |
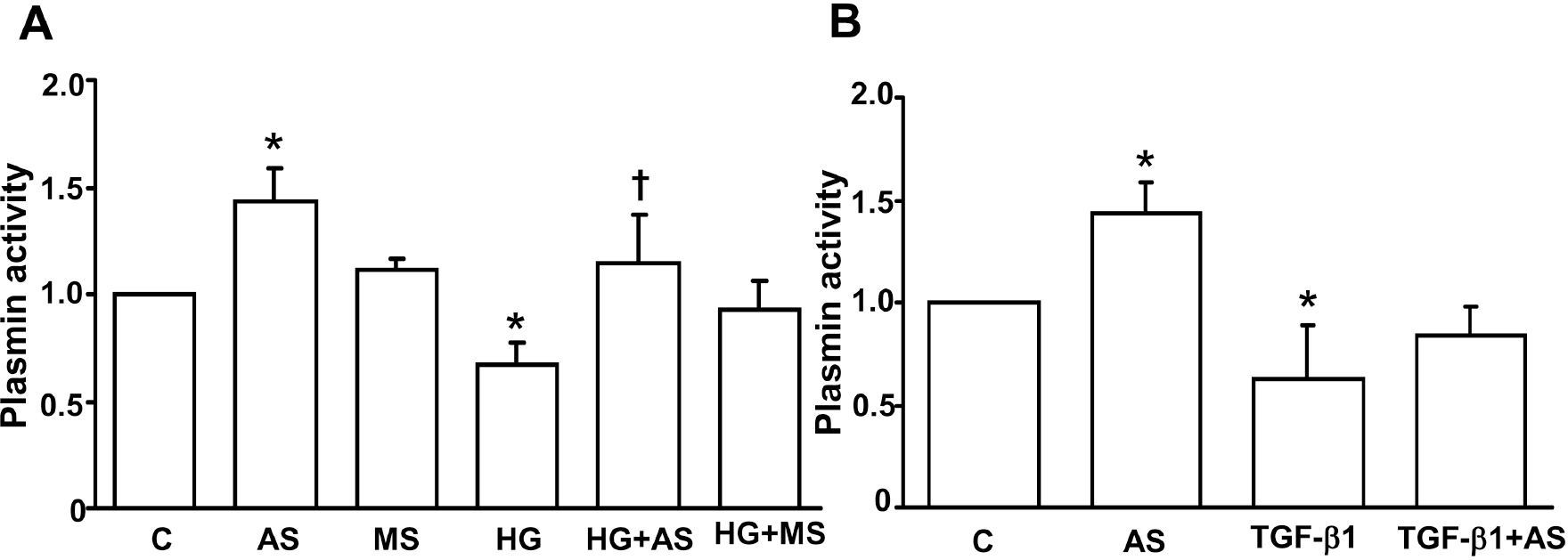 | Fig. 2.Effect of PAI-1 antisense ODN on HG- (A) and TGF-β1- (B) suppressed plasmin activity in mesangial cells. Growth arrested and synchronized primary rat mesangial cells were stimulated with 30 mM high D-glucose (HG) (A) or 2 ng/ml of TGF-β 1 (B) for 48 hours after PAI-1 antisense or mis-match ODN transfection. Plasmin activity in the media was measured as described in the Methods. Data are presented as means±SE of 4 experiments. ∗p<0.05 vs control without HG or TGF-β 1, p<0.05 vs HG or TGF-β 1, C: control without HG or TGF-β1, AS: PAI-1 antisense ODN, MS: PAI-1 mis-match sense ODN. |
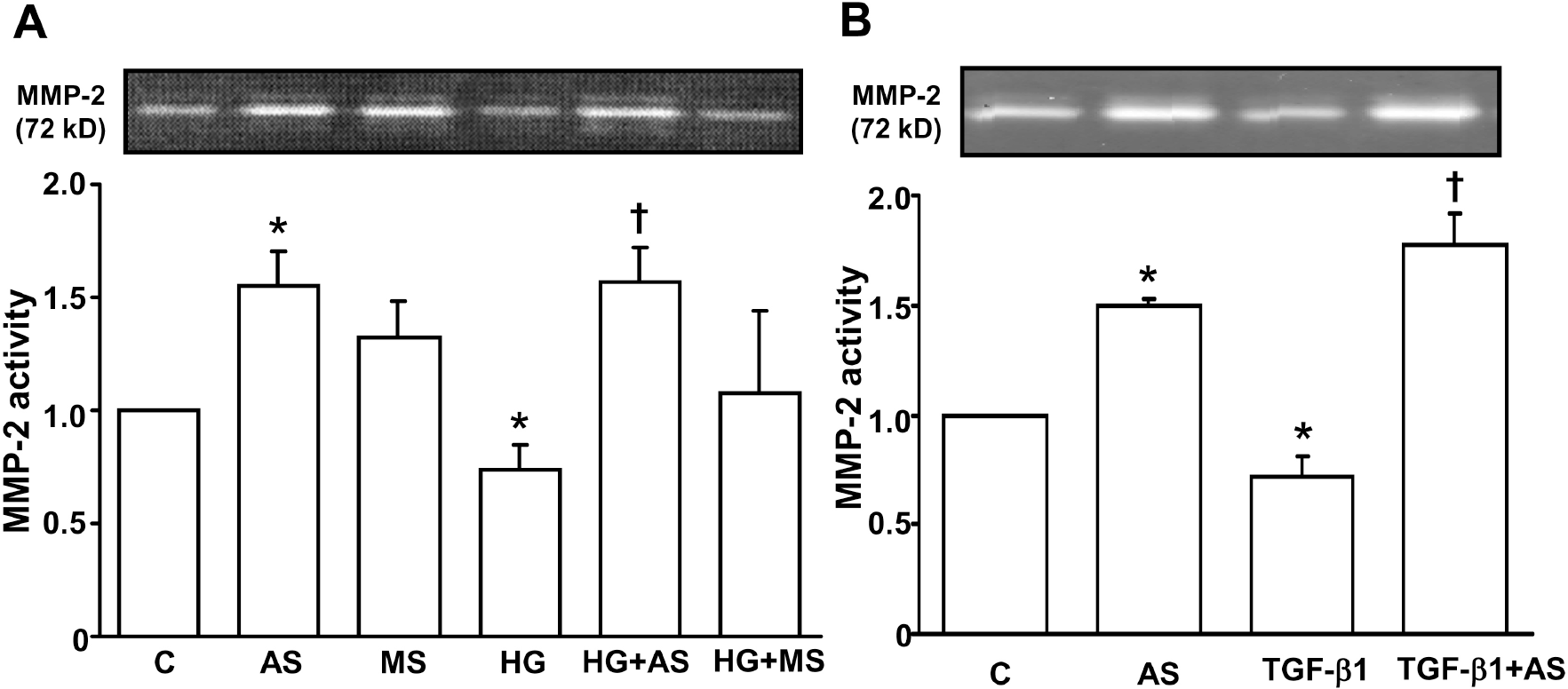 | Fig. 3.Effect of PAI-1 antisense ODN on HG- (A) and TGF-β1- (B) suppressed MMP-2 activity in mesangial cells. Growth arrested and synchronized primary rat mesangial cells were stimulated with 30 mM high D-glucose (HG) (A) or 2 ng/ml of TGF-β1 (B) for 48 hours after PAI-1 antisense or mis-match ODN transfection. MMP-2 activity was detected by gelatin zymography as described in the Methods. Data are presented as means±SE of 4 experiments. ∗p<0.05 vs control without HG or TGF-β1, †p<0.05 vs HG or TGF-β1, C: control without HG or TGF-β1, AS: PAI-1 antisense ODN, MS: PAI-1 mis-match sense ODN. |
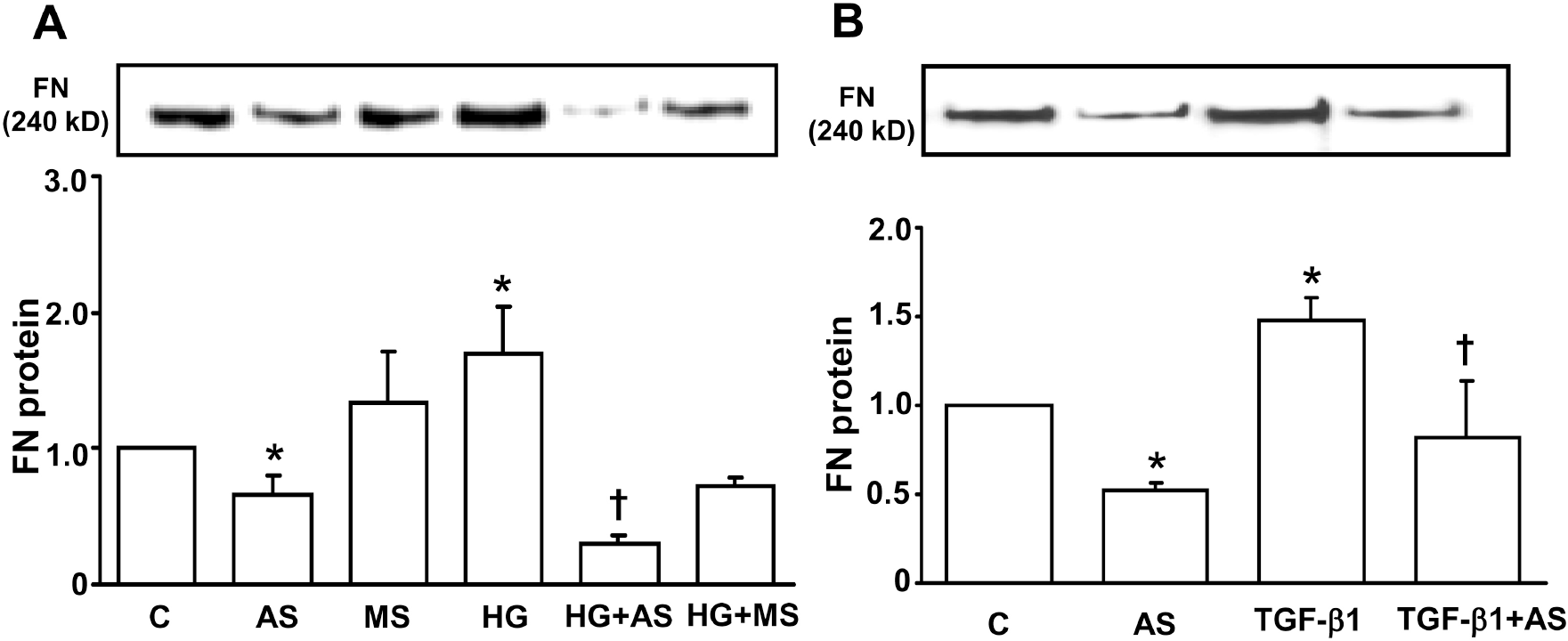 | Fig. 4.Effect of PAI-1 antisense ODN on HG- (A) and TGF-β1- (B) increased fibronectin secretion in mesangial cells. Growth arrested and synchronized primary rat mesangial cells were stimulated with 30 mM high D-glucose (HG) (A) or 2 ng/ml of TGF-β1 (B) for 48 hours after PAI-1 antisense or mis-match sense ODN transfection. Fibronectin protein was detected by Western blot analysis as described in the Methods. Data are presented as means±SE of 4 experiments. ∗p<0.05 vs control without HG or TGF-β1, †p<0.05 vs HG or TGF-β1, C: control without HG or TGF-β1, AS: PAI-1 antisense ODN, MS: PAI-1 mis-match sense ODN. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download