Abstract
The serine/threonine kinase Akt has been shown to play a role of multiple cellular signaling pathways and act as a transducer of many functions initiated by growth factor receptors that activate phosphatidylinositol 3-kinase (PI3K). It has been reported that phosphorylated Akt activates eNOS resulting in the production of NO and that NO stimulates soluble guanylate cyclase (sGC), which results in accumulation of cGMP and subsequent activation of the protein kinase G (PKG). It has been also reported that PKG activates PI3K/Akt signaling. Therefore, it is possible that PI3K, Akt, eNOS, sGC, and PKG form a loop to exert enhanced and sustained activation of Akt. However, the existence of this loop in eNOS-expressing cells, such as endothelial cells or astrocytes, has not been reported. Thus, we examined a possibility that Akt phosphorylation might be enhanced via eNOS/sGC/PKG/PI3K pathway in astrocytes in vivo and in vitro. Phosphorylation of Akt was detected in astrocytes after KA treatment and was maintained up to 72 h in mouse hippocampus. 2 weeks after KA treatment, astrocytic Akt phosphorylation was normalized to control. The inhibition of eNOS, sGC, and PKG significantly decreased Akt and eNOS phosphorylation induced by KA in astrocytes. In contrast, the decreased phosphorylation of Akt and eNOS by eNOS inhibition was significantly reversed with PKG activation. The above findings in mouse hippocampus were also observed in primary astrocytes. These data suggest that Akt/eNOS/sGC/PKG/PI3K pathway may constitute a loop, resulting in enhanced and sustained Akt activation in astrocytes.
Go to : 
References
1. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999; 399:601–605.

2. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999; 399:597–601.

3. Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999; 1411:334–350.

4. Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003; 35:21–27.

5. Kawasaki K, Smith RS Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003; 23:5726–5737.

6. Ha KS, Kim KM, Kwon YG, Bai SK, Nam WD, Yoo YM, Kim PK, Chung HT, Billiar TR, Kim YM. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. Faseb J. 2003; 17:1036–1047.

7. Chang MS, Lee WS, Chen BC, Sheu JR, Lin CH. YC-1-induced cyclooxygenase-2 expression is mediated by cGMP-dependent activations of Ras, phosphoinositide-3-OH-kinase, Akt, and nuclear factor-kappaB in human pulmonary epithelial cells. Mol Pharmacol. 2004; 66:561–571.
8. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357.
9. Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993; 52:115–134.

10. Byun J, Lee S, Jeon S, Kwon Y, Lee HJ, Kim S, Kim Y, Kim M, Chun W. Kainic acid-induced neuronal death is attenuated by aminoguanidine but aggravated by L-NAME in mouse hippocampus. Korean J Physiol Pharmacol. 2009; 13:265–271.

11. Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000; 58:1026–1034.

12. Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002; 22:8911–8921.

13. Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the proteinkinase-B pathway. Nature. 1998; 396:584–587.

14. Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998; 273:32377–32379.

15. Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000; 275:10761–10766.

16. Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999; 401:86–90.
17. Cen X, Nitta A, Ohya S, Zhao Y, Ozawa N, Mouri A, Ibi D, Wang L, Suzuki M, Saito K, Ito Y, Kawagoe T, Noda Y, Ito Y, Furukawa S, Nabeshima T. An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway. J Neurosci. 2006; 26:3335–3344.

18. Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002; 277:35920–35931.

19. Lee SH, Chun W, Kong PJ, Han JA, Cho BP, Kwon OY. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal. Res. 2006; 40:79–85.

20. Lee KY, Ito K, Hayashi R, Jazrawi EP, Barnes PJ, Adcock IM. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J Immunol. 2006; 176:603–615.
21. Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003; 23:1174–1182.
22. Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003; 39:13–22.
23. Vivien D, Bernaudin M, Buisson A, Divoux D, MacKenzie ET, Nouvelot A. Evidence of type I and type II transforming growth factor-beta receptors in central nervous tissues: changes induced by focal cerebral ischemia. J Neurochem. 1998; 70:2296–2304.
24. Buisson A, Nicole O, Docagne F, Sartelet H, Mackenzie ET, Vivien D. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. Faseb J. 1998; 12:1683–1691.
25. Docagne F, Nicole O, Gabriel C, Fernandez-Monreal M, Lesne S, Ali C. Smad3-dependent induction of plasminogen activator inhibitor-1 in astrocytes mediates neuroprotective activity of transforming growth factor-beta 1 against NMDA-induced necrosis. Mol Cell Neurosci. 2002; 21:634–644.
26. Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004; 172:7015–7023.
Go to : 
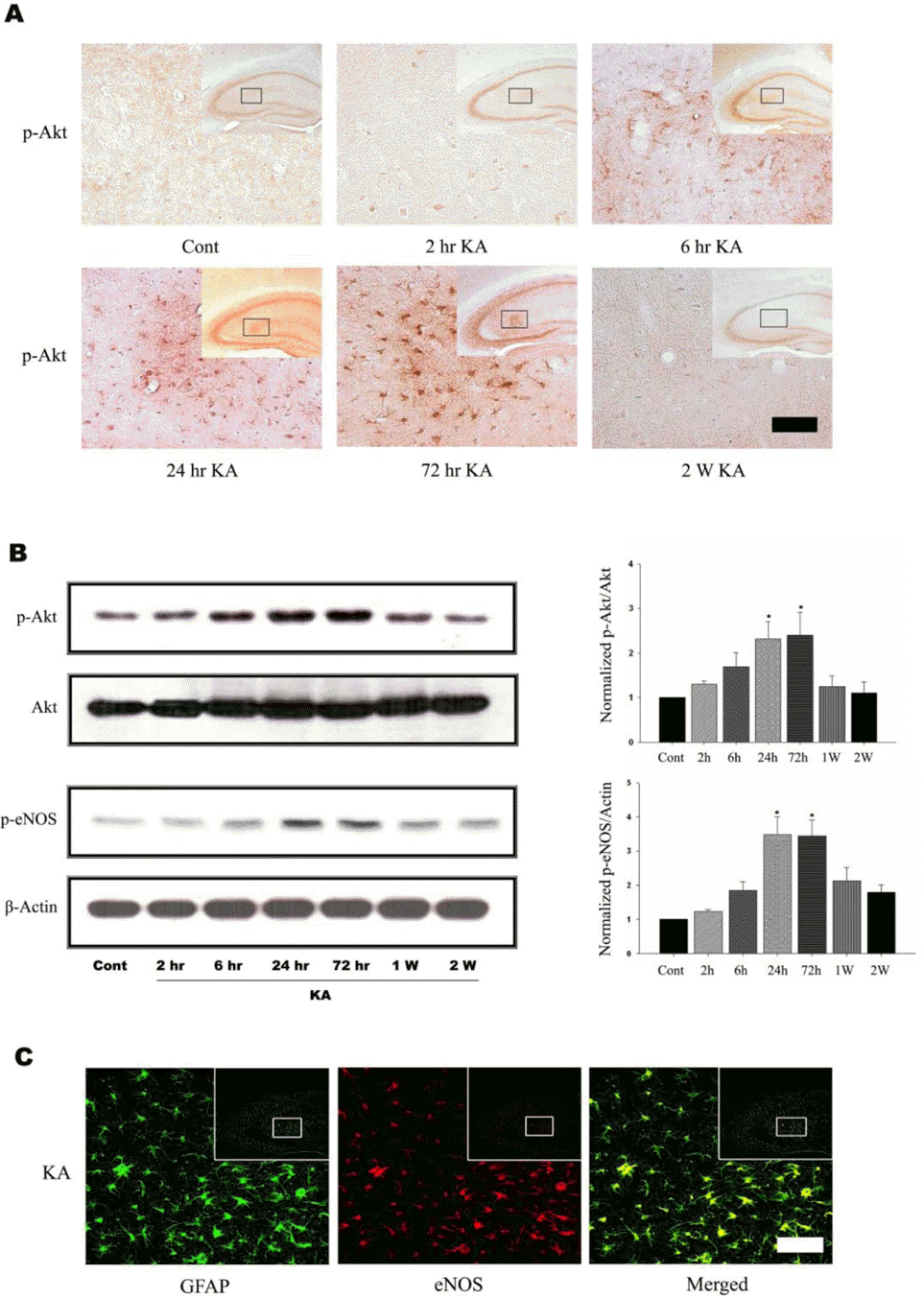 | Fig. 1.KA induces Akt and eNOS phosphorylation in mouse hippocampus. (A) Time course of KA-induced Akt phosphorylation in astrocytes. Increased p-Akt immunoreactivity was observed predominantly in astrocytes in the hippocampus 6 h after KA treatment and maintained up to 72 h. Inset at right upper corner shows the position of the enlarged image of CA3 region. (B) Representative immunoblots (left) and quantitative analysis (right) of Akt and eNOS phosphorylation. The levels of phosphorylation of Akt at Ser473 and eNOS at Ser1177 were measured after KA injection. Phosphorylation of Akt and eNOS increased up to 3 days, and then normalized 2 weeks after KA injection. (C) Confocal image of astrocytic eNOS in the hippocampus. Double immunofluorescence staining was carried out with antibodies to eNOS and GFAP, an astrocytic marker. eNOS immunoreactivity appeared to co-localize with that of GFAP. Data represent three independent experiments and were expressed mean±SD. ∗p<0.05 indicate statistically significant difference from control group. Scale bar: 50μm. |
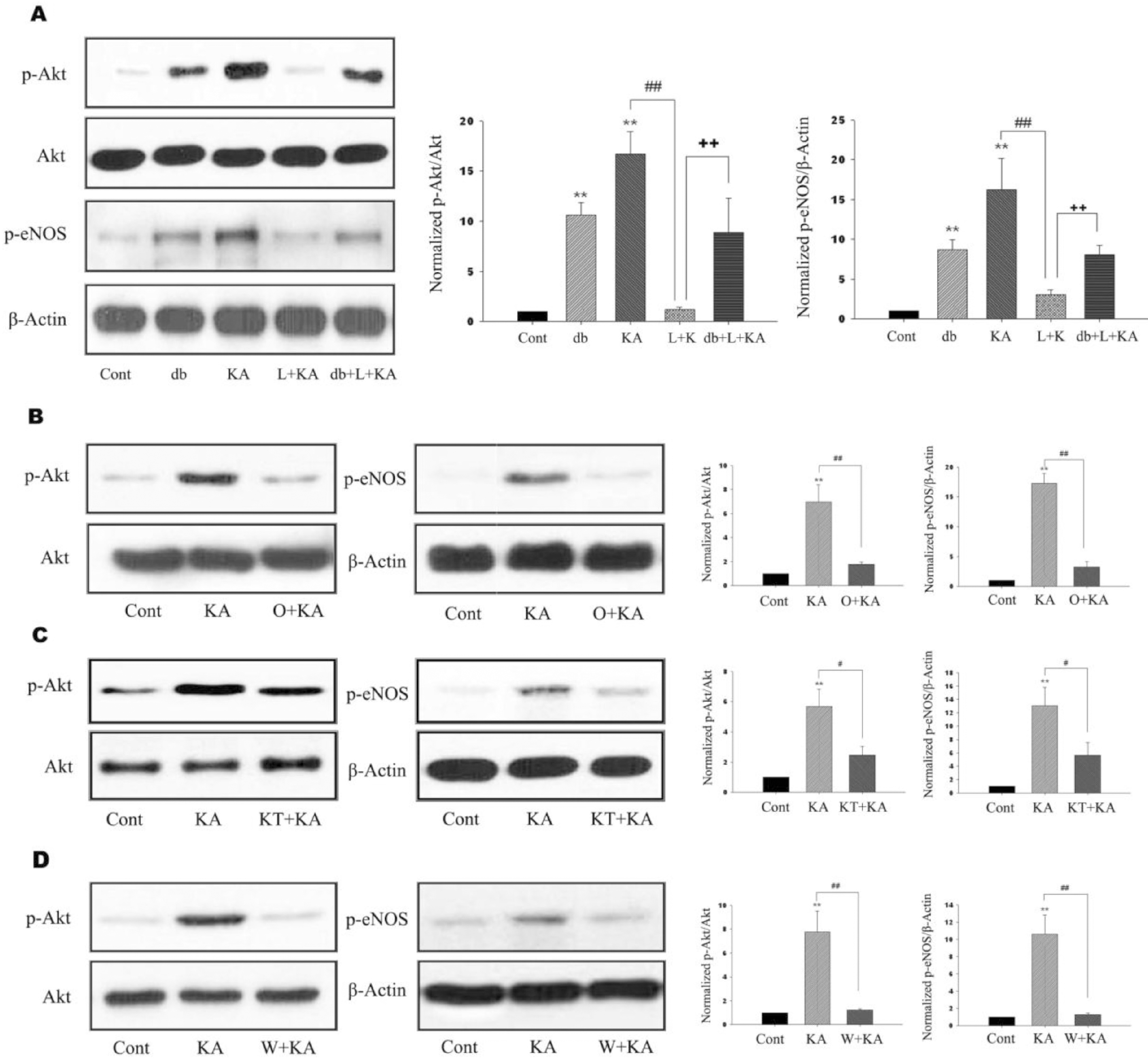 | Fig. 2.Effects of L-NAME, ODQ, KT-5823, and wortmannin on phosphorylation of Akt and eNOS. (A) Representative immunoblots (left) and quantitative analyses (right) of effects of db-cGMP and L-NAME on phosphorylation of Akt and eNOS. db-cGMP and KA exhibited significantly increased Akt and eNOS phosphorylation (db, KA). However, L-NAME significantly decreased KA-induced phosphorylation of Akt and eNOS (L+ KA). Further, co-treatment of db- cGMP attenuated L-NAME-mediated decrease of Akt and eNOS phosphorylation (db+L+KA). (B∼D) Effects of ODQ, KT-5823, and wortmannin on phosphorylation of Akt and eNOS. Representative immunoblots (left) and quantitative analyses (right) showed that KA-induced phosphorylation of Akt and eNOS was significantly decreased with ODQ, KT-5823, and wortmannin (O+KA, KT+KA, W+KA). Data represent three independent experiments and were expressed mean±SD. ∗∗p< 0.01 indicates statistically significant difference from the control group. # indicates p<0.05, ## and ++ indicate p <0.01 between indicated groups. |
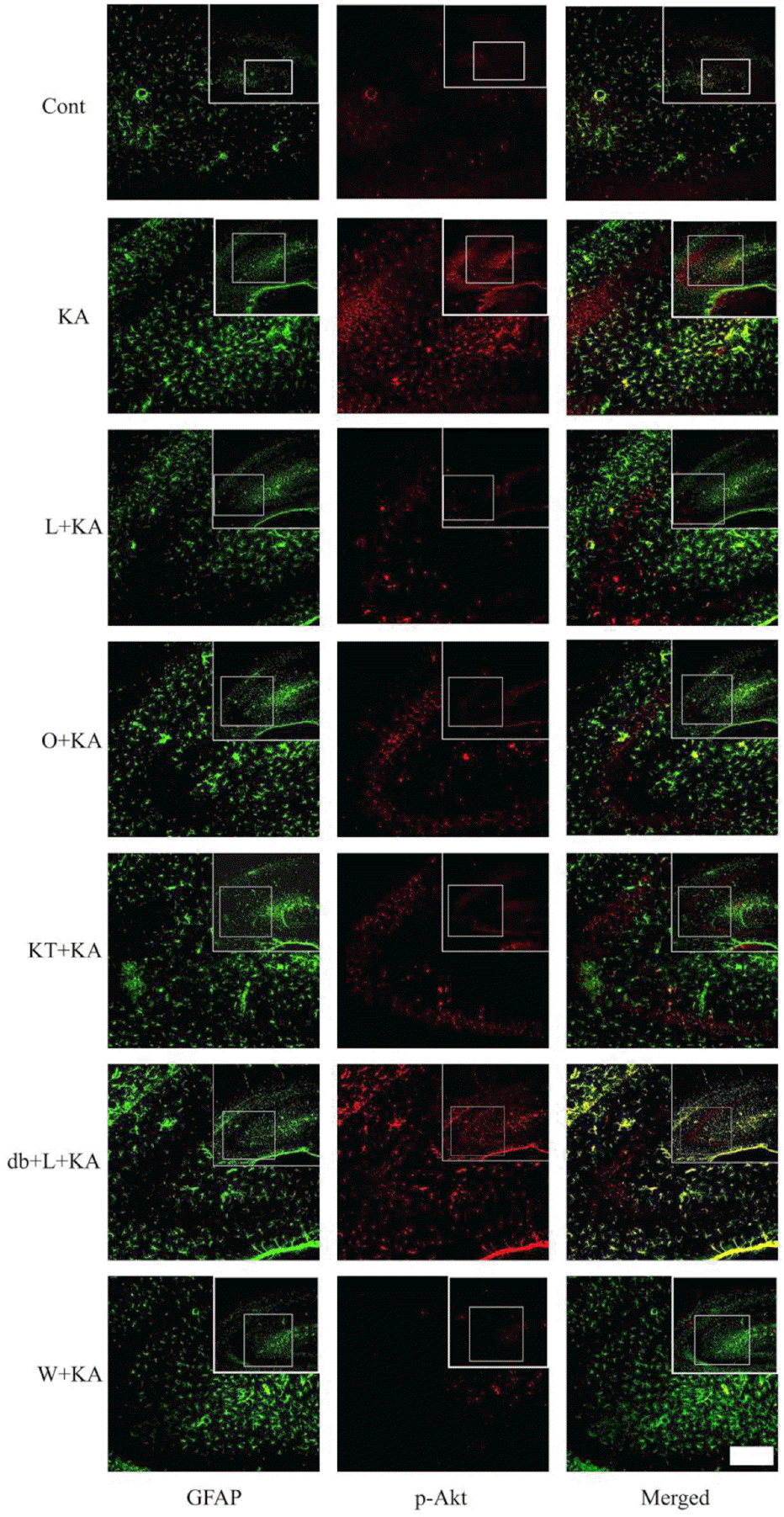 | Fig. 3.Confocal images of astrocytic p-Akt immunoreactivity indicate in hippocampus upon the absence or presence of chemicals prior to KA treatment. To determine whether Akt phosphorylation occurs in astrocytes, double immunofluorescence staining was carried out with antibodies to phosphor-Akt and GFAP. KA per se exhibited increased Akt phosphorylation (KA), which was found to co-localize mainly with GFAP in astrocytes in the hippocampus. L-NAME, ODQ, and KT-5823 noticeably attenuated KA-induced Akt phosphorylation (L+KA, O+KA, & KT+KA). However, db-cGMP, a PKG activator, exhibited restoration of decreased Akt phosphorylation, which was caused by L-NAME, indicating that PKG is involved in Akt activation (db+L+KA). Wortmannin, a PI3K inhibitor, also decreased Akt phosphorylation in astrocytes (W+KA). Inset at right upper corner shows the position of the enlarged image of CA3 region. Scale bar: 50μm. |
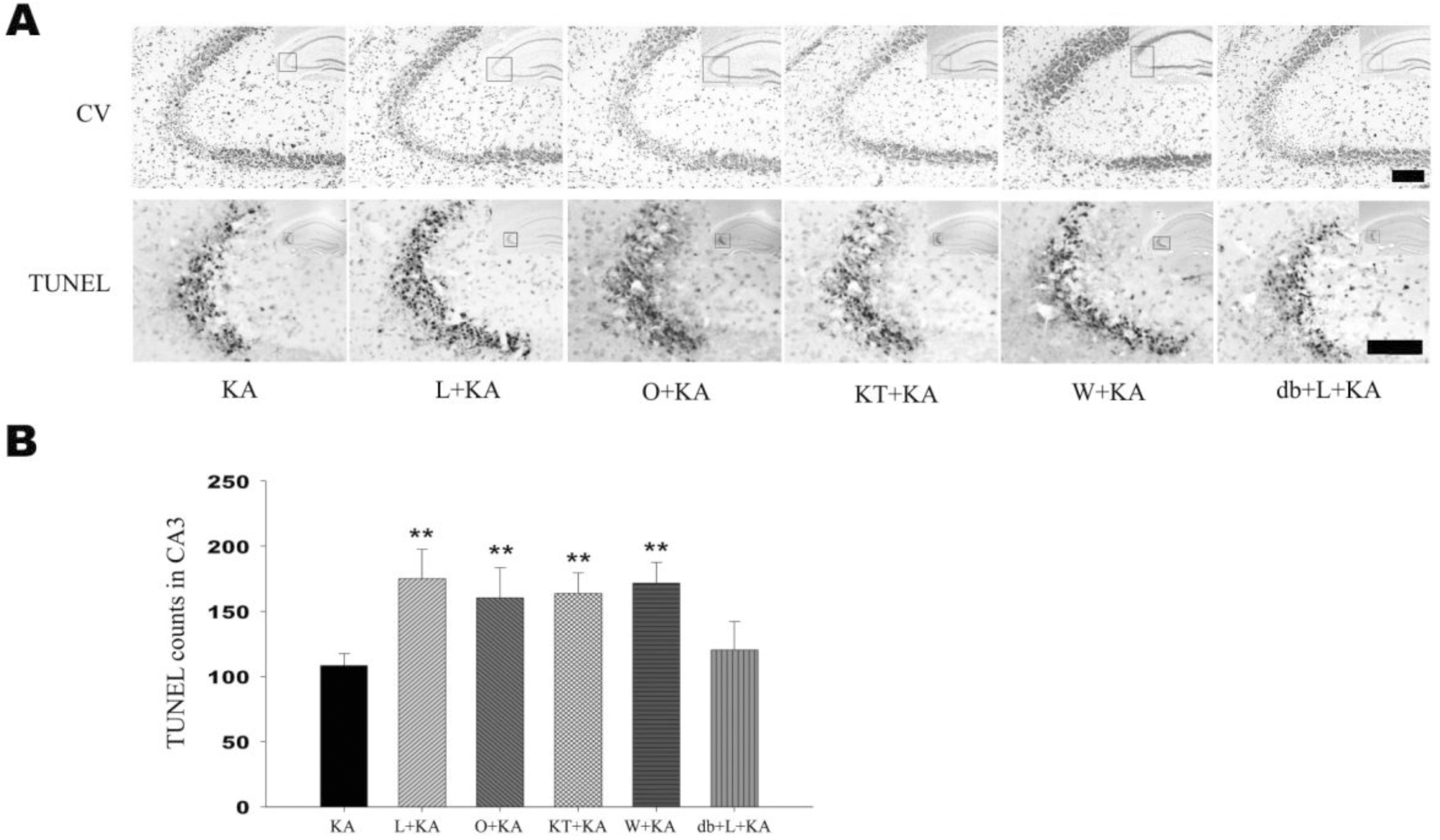 | Fig. 4.Effect of L-NAME, ODQ, KT-5823, and wortmannin on neuronal cell death. Representative (A) and quantitative analysis (B) of neuronal cell death using cresyl violet and TUNEL stainings. In CA3 subfield, KA-induced neuronal loss was aggravated with pretreatment of L-NAME, ODQ, KT-5823, and wortmannin in cresyl violet staining. Considerable number of TUNEL-positive neurons appeared with KA treatment within CA3 region (KA) and the number of TUNEL-positive neurons were significantly increased with pre-treatment of L-NAME, ODQ, KT-5823, and wortmannin (L+KA, O+KA, KT+KA, W+KA). Co-treatment of db-cGMP with L-NAME showed attenuation on L-NAME-induced aggravation of cell death, although not significant (db+L+KA). Inset at right upper corner shows the position of the enlarged image of CA3 region. Data represent three independent experiments and were expressed mean±SD. ∗∗p<0.01 indicates statistically significant difference from the KA-only treated group. Scale bar: 100 mm. |
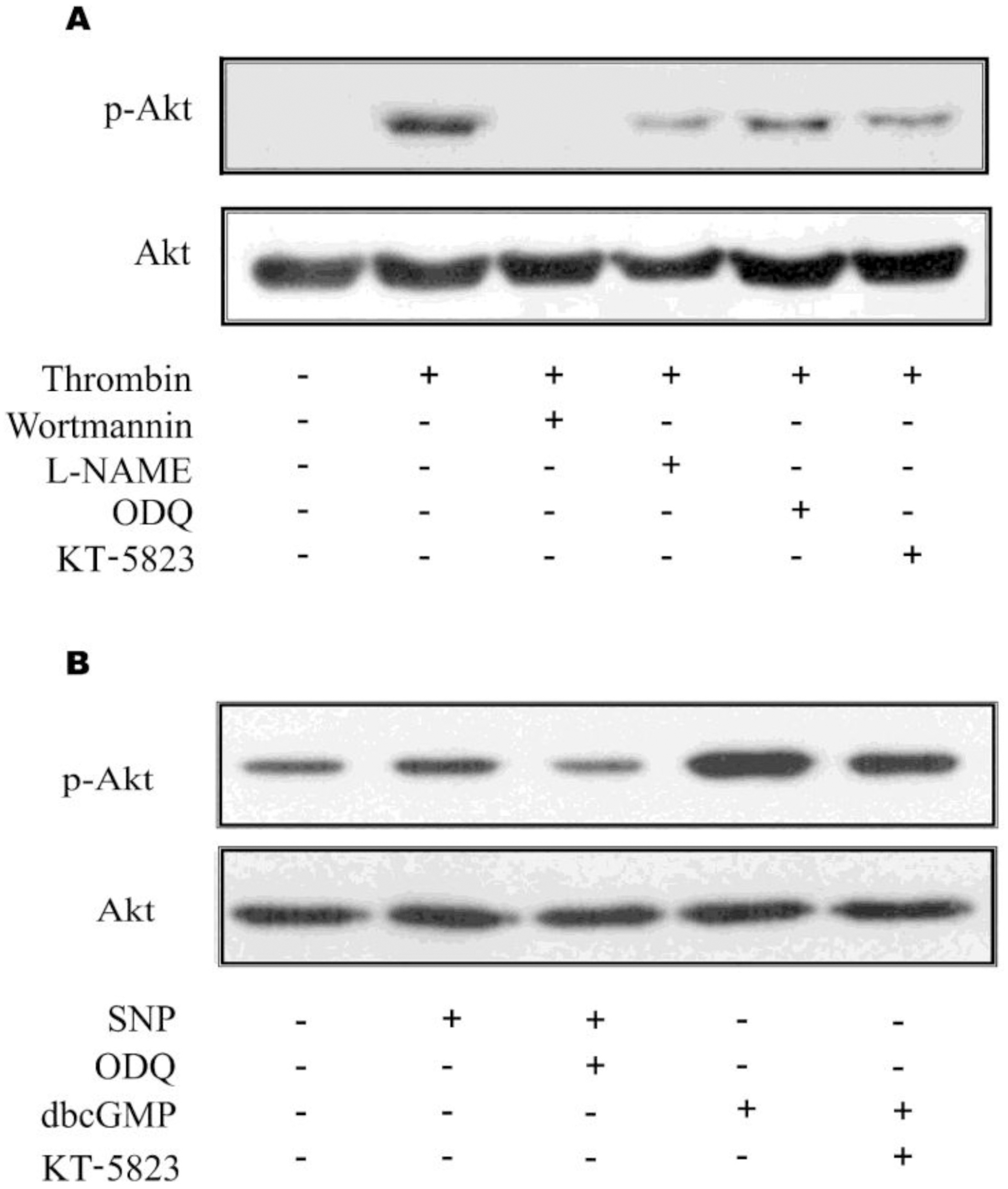 | Fig. 5.
In situ verification of Akt activation in primary astrocytes. Akt phosphorylation was evaluated 16 h after thrombin or other treatments. (A) Phosphorylation of Akt and its inhibition were increase in primary culture astrocytes. Thrombin exhibited increased Akt phosphorylation in primary astrocytes and thrombin-mediated increased Akt phosphorylation was attenuated with inhibitors, which suppress each step of Akt/eNOS/sGC/PKG/PI3K pathway. (B) Akt phosphorylation was increased by exogenous NO by SNP. Treatment of SNP resulted in increased phosphorylation of astrocytic Akt and db-cGMP, a PKG activator, resulted in increased Akt activation, suggesting that the pathway of Akt/eNOS/sGC/PKG/PI3K be present in astrocytes. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download