Abstract
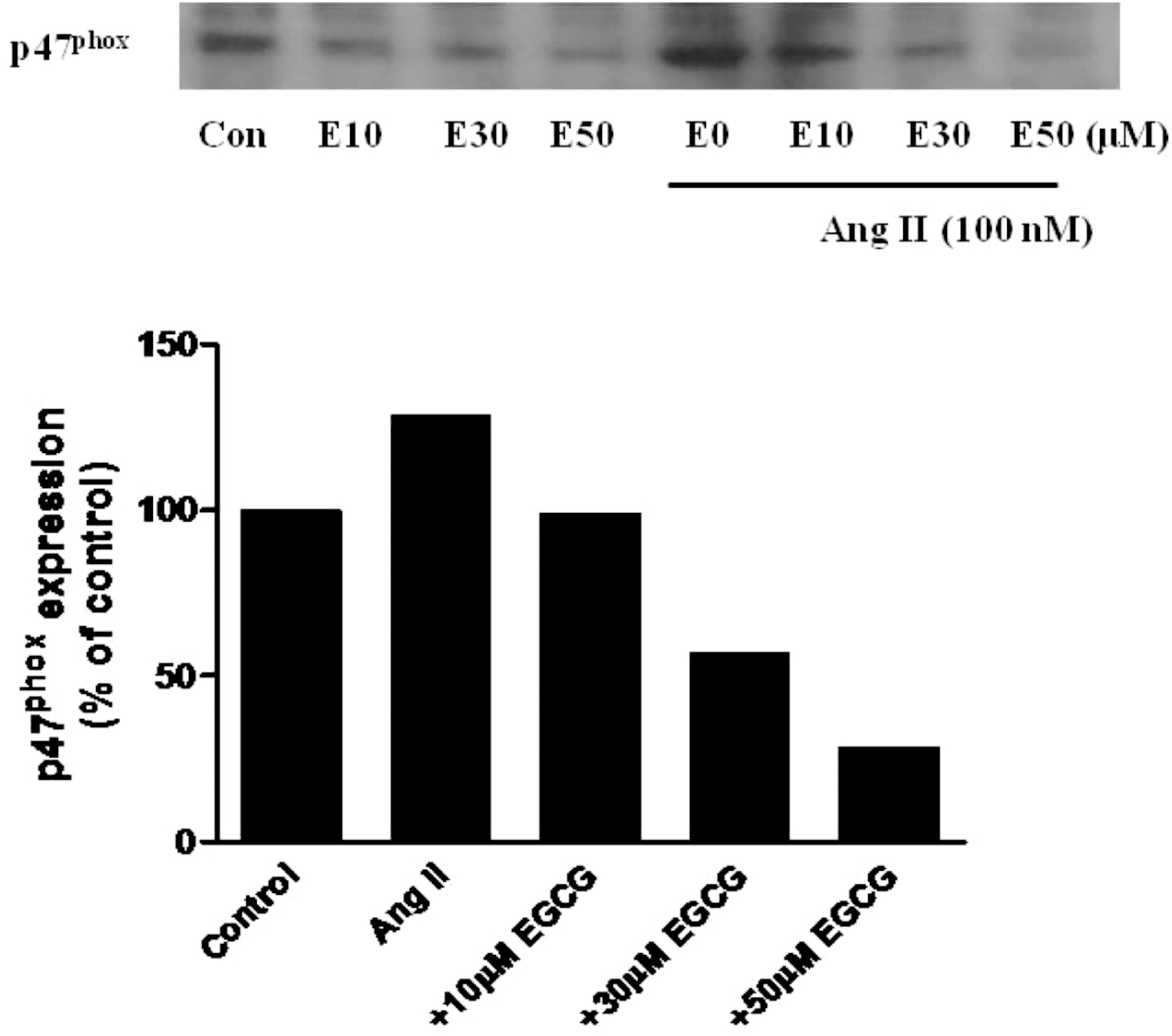
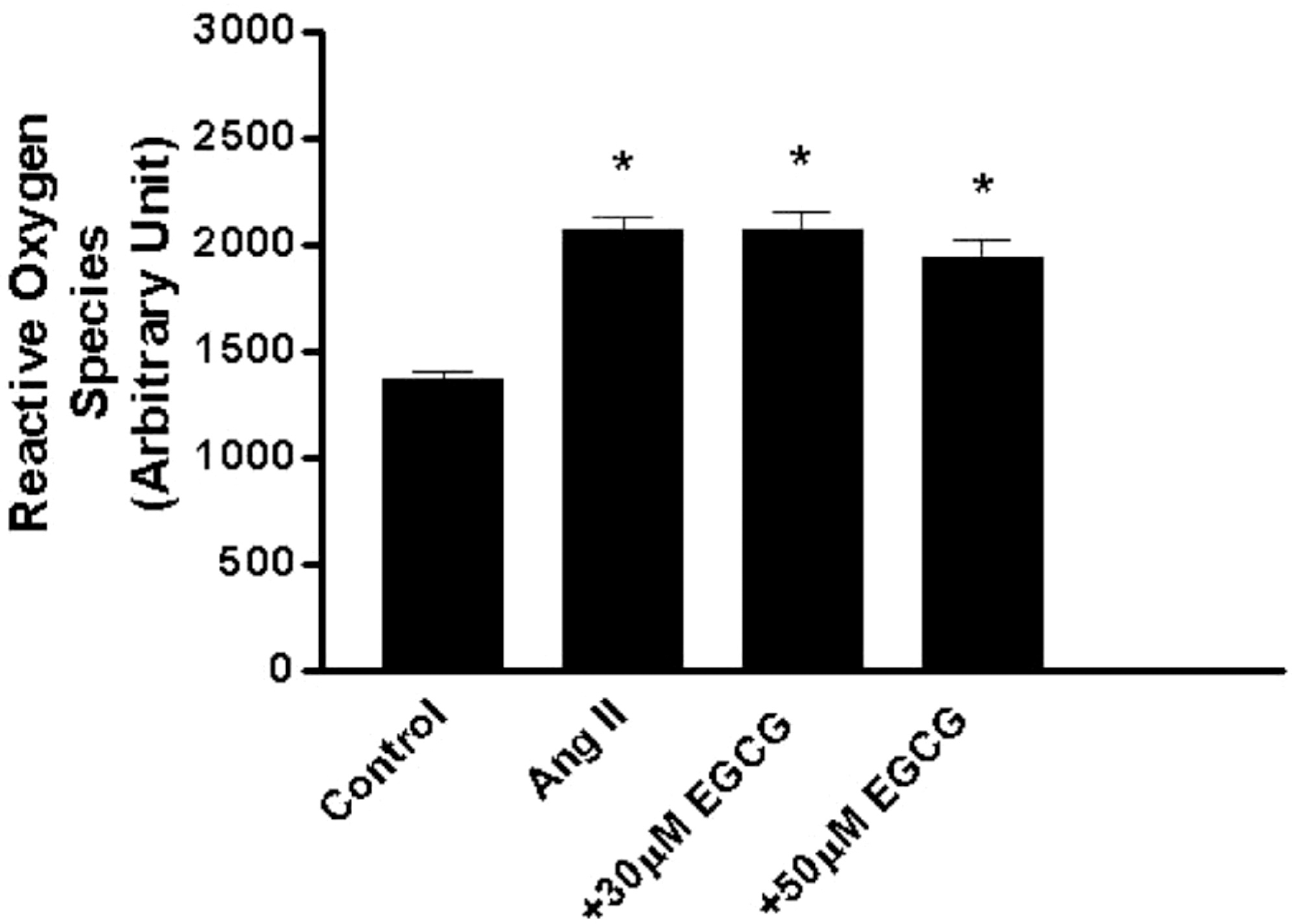
Vascular NADPH oxidase plays a pivotal role in producing superoxide in endothelial cells and thus acts in the initiation and development of inflammatory cardiovascular diseases such as atherosclerosis. Epigallocatechin-3-gallate (EGCG), the major catechin derived from green tea, has multiple beneficial effects for treating cardiovascular disease but the effect of EGCG on the expression of vascular NADPH oxidase remains unknown. In this study, we investigated the mechanism(s) by which EGCG might inhibit the expression of subunits of NADPH oxidase, namely p47phox, p67phox and p22phox, induced by angiotensin II (Ang II) in human umbilical vein endothelial cells. Ang II increased the expression levels of p47phox, p67phox, and p22phox, but EGCG counteracted this effect on p47phox. Moreover, EGCG did not affect the production of reactive oxygen species induced by Ang II. These data suggest a novel mechanism whereby EGCG might provide direct vascular benefits for treating inflammatory cardiovascular diseases.
Go to : 
References
1. Rueckschloss U, Duerrschmidt N, Morawietz H. NADPH oxidase in endothelial cells: impact on atherosclerosis. Antioxid Redox Signal. 2003; 5:171–180.

2. Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008; 45:1340–1351.

3. Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NADPH oxidase. Mol Cell Biochem. 2004; 264:85–97.

4. Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A, Daniel G. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999; 44:215–222.

5. Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappa B activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000; 20:645–651.
6. Wassmann S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006; 24:S15–S21.

7. Gao L, Mann GE. Vascular NADPH oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovasc Res. 2009; 82:9–20.
8. Hu C, Dandapat A, Chen J, Liu Y, Hermonat PL, Carey RM, Mehta JL. Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis. 2008; 199:288–294.

9. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751.
10. Sasazuki S, Kodama H, Yoshimasu K, Liu Y, Washio M, Tanaka K, Tokunaga S, Kono S, Arai H, Doi Y, Kawano T, Nakagaki O, Takada K, Koyanagi S, Hiyamuta K, Nii T, Shirai K, Ideishi M, Arakawa K, Mohri M, Takeshita A. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Ann Epidemiol. 2000; 10:401–408.

11. Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, Stangl K, Baumann G, Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced vcam-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004; 316:659–665.

12. Chae YJ, Kim CH, Ha TS, Hescheler J, Ahn HY, Sachinidis A. Epigallocatechin-3-O-gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways. Cell Physiol Biochem. 2007; 20:859–866.

13. Ahn HY, Xu Y, Davidge ST. Epigallocatechin-3-O-gallate inhibits TNFα-induced monocyte chemotactic protein-1 production from vascular endothelial cells. Life Sciences. 2008; 82:964–968.
14. Niemiec P, Zak I. Vascular NADPH oxidases-role in the pathogenesis of atherosclerosis. Postepy Biochem. 2005; 51:1–11.
16. Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002; 32:1116–1122.
17. Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NADPH oxidase. Circulation. 2004; 109:227–233.

18. Dworakowski R, Alom-Ruiz SP, Shah AM. NADPH oxidase-derived reactive oxygen species in the regulation of endothelial phenotype. Pharmacol Rep. 2008; 60:21–28.
19. Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov. 2007; 2:23–27.

20. Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002; 40:511–515.
21. Dorenkamp M, Riad A, Stiehl S, Spillmann F, Westermann D, Du J, Pauschinger M, Noutsias M, Adams V, Schultheiss HP, Tschope C. Protection against oxidative stress in diabetic rats: role of angiotensin AT(1) receptor and beta 1-adrenoceptor antagonism. Eur J Pharmacol. 2005; 520:179–187.

22. Yao R, Cheng X, Chen Y, Xie JJ, Yu X, Liao MY, Ding YJ, Tang TT, Liao YH. Molecular mechanisms of irbesartan suppressing atherosclerosis in high cholesterol-diet apolipoprotein E knock-out mice. Int J Cardiol. 2010; 139:113–122.
23. Alvarez E, Rodino-Janeiro BK, Ucieda-Somoza R, Gonzalez-Juanatey JR. Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol. 2010; 55:203–212.

24. Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: Part 1. Review of noncancer health benefits. J Altern Complement Med. 2005; 11:521–528.
25. Zheng Y, Song HJ, Yun SH, Chae YJ, Kim CH, Ha TS, Sachinidis A, Ahn HY, Davidge ST. Inhibition of angiotensin II-induced vascular smooth muscle cell hypertrophy by different catechins. Korean J Physiol Pharmacol. 2005; 9:117–123.
26. Ramesh E, Geraldine P, Thomas PA. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis. Chem Biol Interact. 2010; 183:125–132.

27. Wang CJ, Liu JT, Guo F. (–)-epigallocatechin gallate inhibits endothelin-1-induced C-reactive protein production in vascular smooth muscle cells. Basic Clin Pharmacol Toxicol. 2010; 107:669–675.

28. Nishikawa H, Wakano K, Kitani S. Inhibition of NADPH oxidase subunits translocation by tea catechin EGCG in mast cell. Biochem Biophys Res Commun. 2007; 362:504–509.

29. Wu CH, Wu CF, Huang HW, Jao YC, Yen GC. Naturally occurring flavonoids attenuate high glucose-induced expression of proinflammatory cytokines in human monocytic THP-1 cells. Mol Nutr Food Res. 2009; 53:984–995.

30. Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol. 2009; 60 Suppl. 4:111–116.
31. Sanchez M, Lodi F, Vera R, Villar IC, Cogolludo A, Jimenez R, Moreno L, Romero M, Tamargo J, Perez-Vizcaino F, Duarte J. Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta. J Nutr. 2007; 137:910–915.
32. Huang SM, Wu CH, Yen GC. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol Nutr Food Res. 2006; 50:1129–1139.

33. Crispo JA, Ansell DR, Piche M, Eibl JK, Khaper N, Ross GM, Tai TC. Protective effects of polyphenolic compounds on oxidative stress-induced cytotoxicity in PC12 cells. Can J Physiol Pharmacol. 2010; 88:429–438.

34. Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CP, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007; 49:362–368.

Go to : 
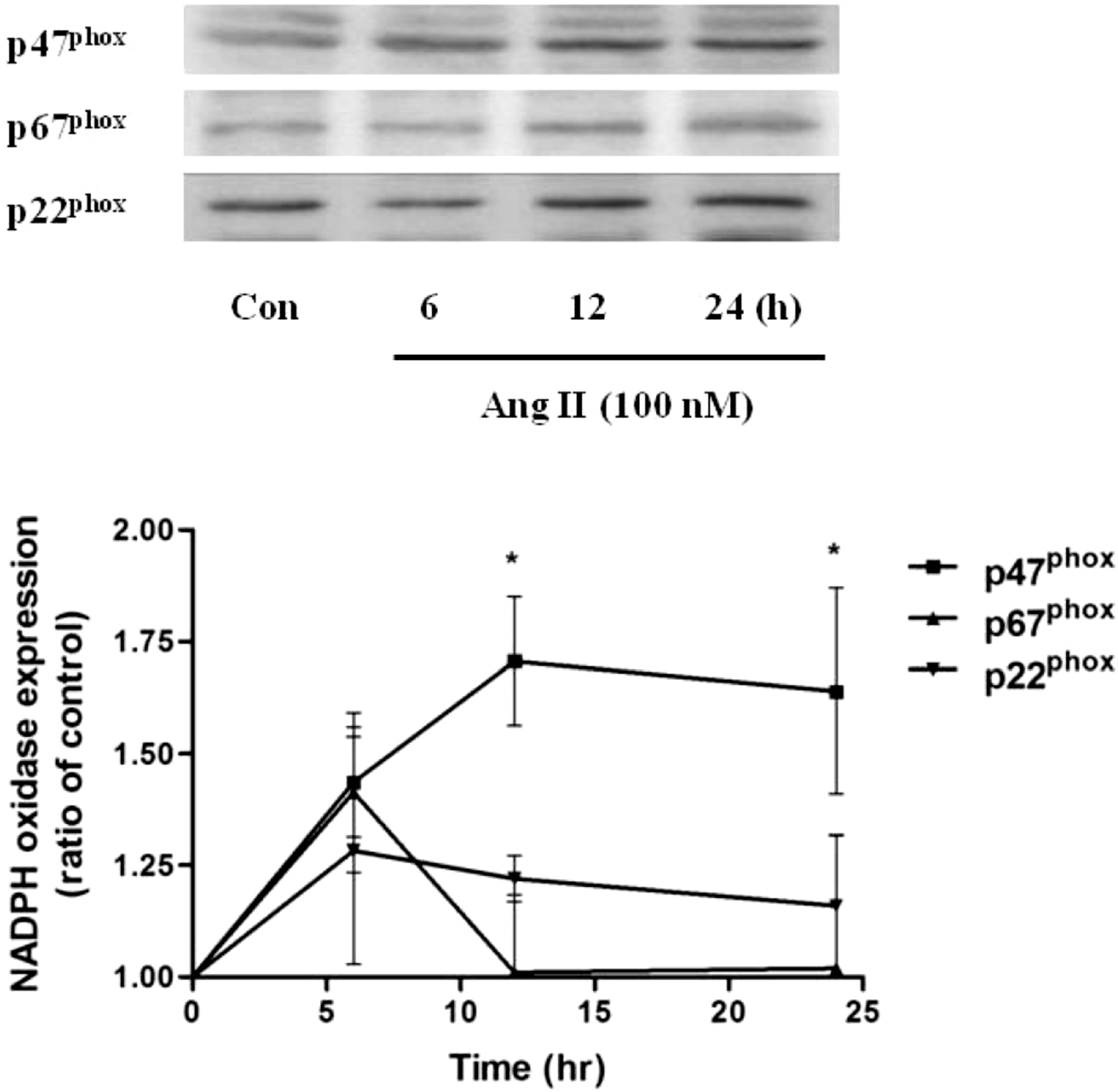 | Fig. 1.Effect of Ang II treatment (100 nM, 0∼24 h) on the expression levels of NADPH oxidase subunits p47phox, p67phox and p22phox in HUVECs. Summary data are shown as the mean±SEM. ∗p<0.05 by Turkey's multiple comparison test. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download