Abstract
Losartan is a selective angiotensin II (Ang II) type 1 (AT1) receptor antagonist which inhibits vascular smooth muscle cells (VSMCs) contraction and proliferation. We hypothesized that losartan may prevent cell proliferation by activating AMP-activated protein kinase (AMPK) in VSMCs. VSMCs were treated with various concentrations of losartan. AMPK activation was measured by Western blot analysis and cell proliferation was measured by MTT assay and flowcytometry. Losartan dose- and time-dependently increased the phosphorylation of AMPK and its downstream target, acetyl-CoA carboxylase (ACC) in VSMCs. Losartan also significantly decreased the Ang II- or 15% FBS-induced VSMC proliferation by inhibiting the expression of cell cycle associated proteins, such as p-Rb, cyclin D, and cyclin E. Compound C, a specific inhibitor of AMPK, or AMPK siRNA blocked the losartan-induced inhibition of cell proliferation and the G0/G1 cell cycle arrest. These data suggest that losartan-induced AMPK activation might attenuate Ang II-induced VSMC proliferation through the inhibition of cell cycle progression.
Go to : 
References
2. Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003; 546:113–120.

3. Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996; 271:27879–27887.

4. Hardie DG. New roles for the LKB1–>AMPK pathway. Curr Opin Cell Biol. 2005; 17:167–173.
5. Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005; 2:21–33.
6. Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997; 273:E1107–E1112.
7. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002; 277:23977–23980.

8. Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005; 25:2488–2494.
9. Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006; 55:2180–2191.

10. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8:1288–1295.

11. Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004; 110:444–451.

12. Asmar R. Targeting effective blood pressure control with angiotensin receptor blockers. Int J Clin Pract. 2006; 60:315–320.

13. Nozawa Y, Matsuura N, Miyake H, Yamada S, Kimura R. Effects of TH-142177 on angiotensin II-induced proliferation, migration and intracellular signaling in vascular smooth muscle cells and on neointimal thickening after balloon injury. Life Sci. 1999; 64:2061–2070.

14. Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995; 95:651–657.

15. Bunkenburg B, Amelsvoort T, Rogg H, Wood JM. Receptor-mediated effects of angiotensin II on growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 1992; 20:746–754.

16. Makita S, Nakamura M, Yoshida H, Hiramori K. Effect of angiotensin II receptor blocker on angiotensin II stimulated DNA synthesis of cultured human aortic smooth muscle cells. Life Sci. 1995; 56::p. L383–PL388.

17. Herbert JM, Delisee C, Dol F, Schaeffer P, Cazaubon C, Nisato D, Chatelain P. Effect of SR 47436, a novel angiotensin II AT1 receptor antagonist, on human vascular smooth muscle cells in vitro. Eur J Pharmacol. 1994; 251:143–150.

18. Okada K, Hirano T, Ran J, Adachi M. Olmesartan medoxomil, an angiotensin II receptor blocker ameliorates insulin resistance and decreases triglyceride production in fructose-fed rats. Hypertens Res. 2004; 27:293–299.

19. Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004; 43:993–1002.
20. Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003; 362:1527–1535.
21. Richey JM, Ader M, Moore D, Bergman RN. Angiotensin II induces insulin resistance independent of changes in interstitial insulin. Am J Physiol. 1999; 277:E920–E926.

22. Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002; 40:872–879.

23. Umeda M, Kanda T, Murakami M. Effects of angiotensin II receptor antagonists on insulin resistance syndrome and leptin in sucrose-fed spontaneously hypertensive rats. Hypertens Res. 2003; 26:485–492.

24. Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension. 2001; 38:884–890.
25. Fujimoto M, Masuzaki H, Tanaka T, Yasue S, Tomita T, Okazawa K, Fujikura J, Chusho H, Ebihara K, Hayashi T, Hosoda K, Nakao K. An angiotensin II AT1 receptor antagonist, telmisartan augments glucose uptake and GLUT4 protein expression in 3T3-L1 adipocytes. FEBS Lett. 2004; 576:492–497.
26. Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the reninangiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003; 42:76–81.

27. Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci. 2004; 117:5479–5487.
28. Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG 4th, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004; 279:43940–43951.
29. Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, Froguel P, Nagai R, Kadowaki T. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord. 2003; 3:243–254.
30. Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008; 283:20186–20197.

31. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006; 574:63–71.
32. Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005; 97:837–844.

Go to : 
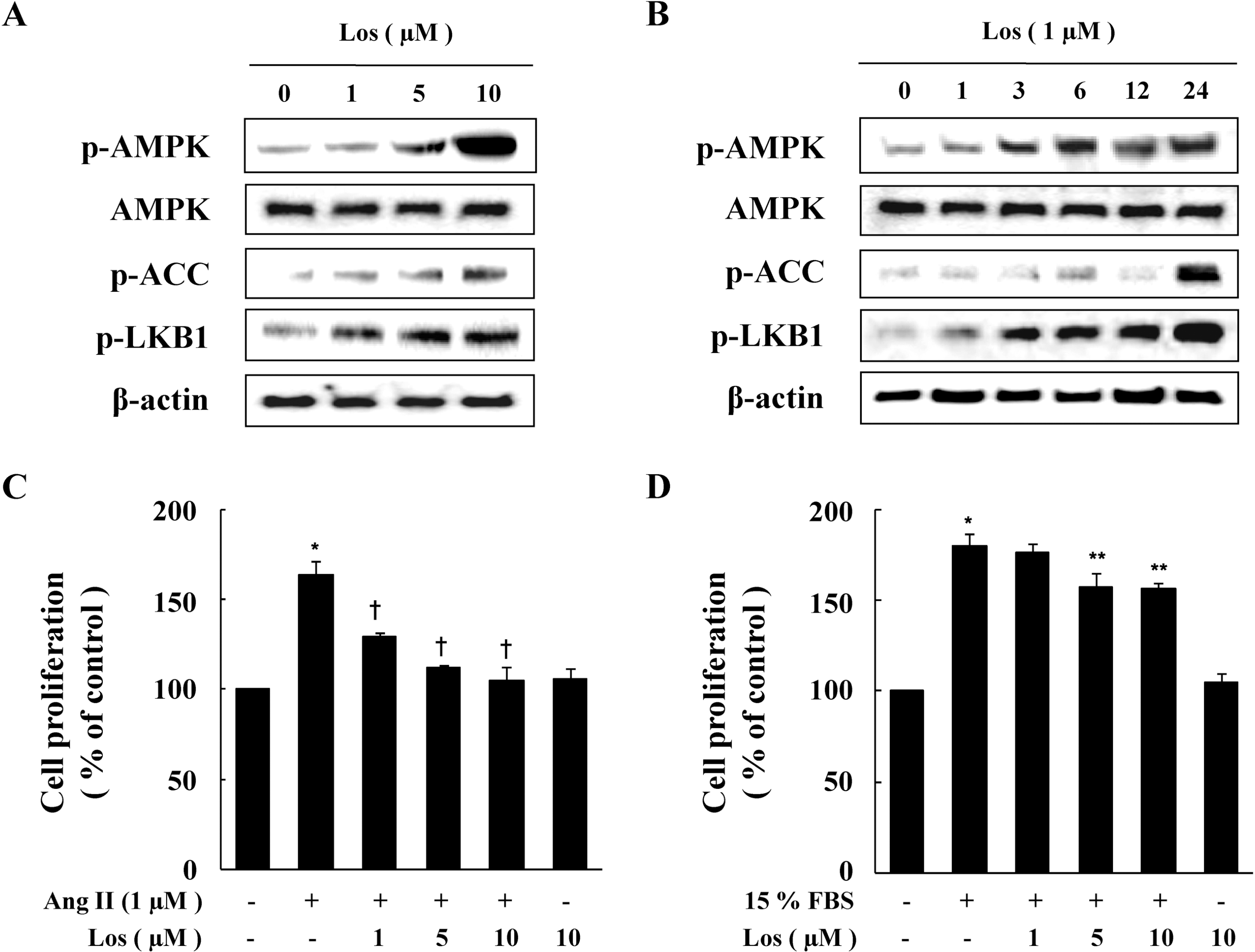 | Fig. 1.Effect of Losartan on the Phosphorylation of AMPK and Cell Proliferation in VSMCs. Cells were treated with the indicated concentration of losartan for 1 hr (A) or for the indicated periods (B). Protein expression of p-AMPK, p-ACC, and p-LKB1 were determined by western blot analysis. Representative blots from three independent experiments are shown. Cells were treated with Ang II (C) or 15% FBS (D) in the presence or absence of indicated concentration of losartan for 48 hrs. Cell proliferation was determined by the MTT assay. Data are represented as the mean±S.E.M (n=4). ∗p value<0.05 compared with control, †p<0.05 compared with Ang II, ∗∗p<0.05 compared with 15% FBS. |
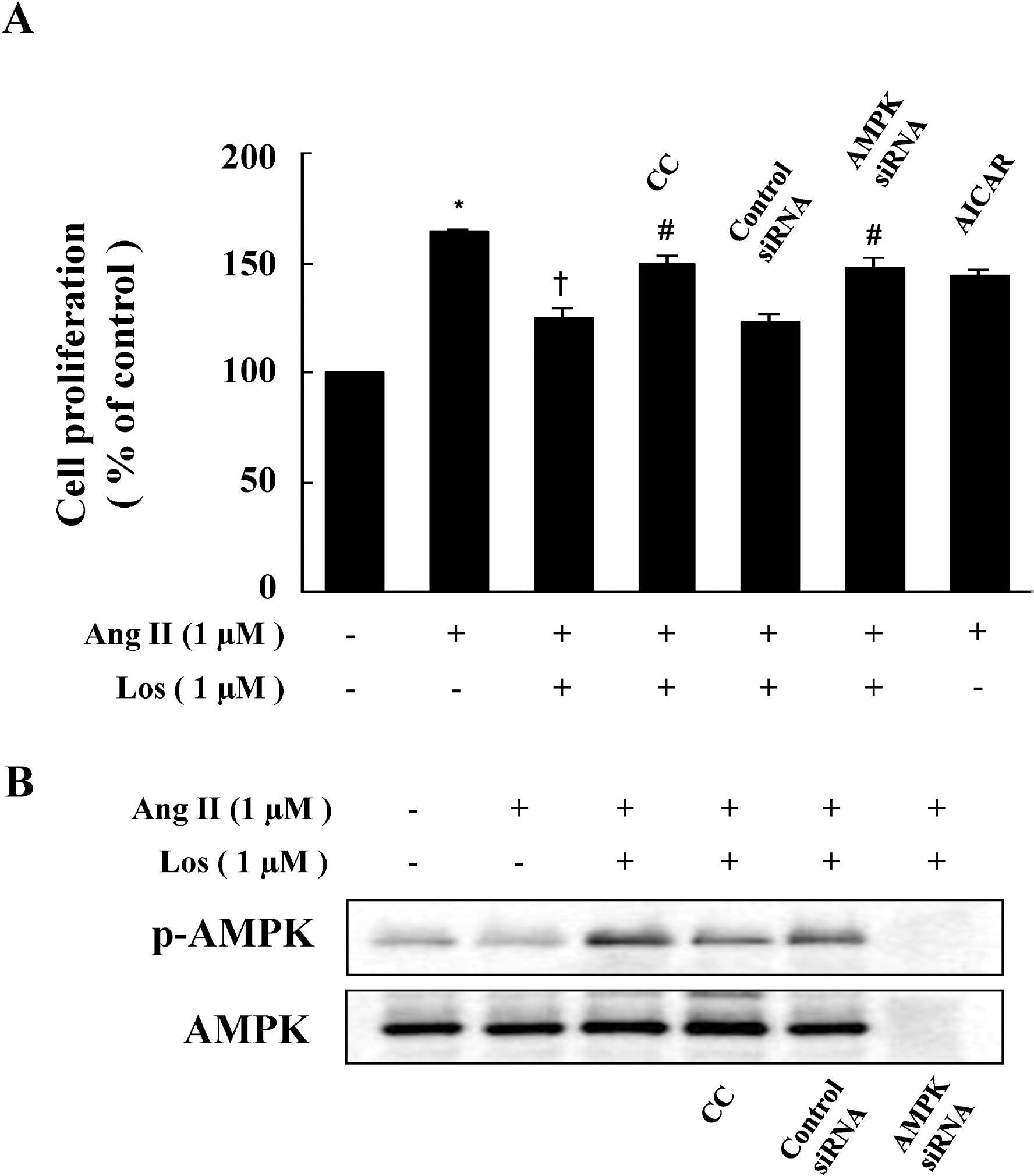 | Fig. 2.Inhibitory Action of Compound C and AMPK siRNA on the Anti-Proliferative Effect of Losartan. (A) Cells were pretreated with compound C (CC) (1 μM) or transfected with control siRNA or AMPK siRNA in the presence of losartan, and then stimulated with Ang II for 48 hrs. Cell proliferation was determined by the MTT assay. Data are represented as the mean±S.E.M (n=4). ∗p<0.05 compared with control, †p<0.05 compared with Ang II, #p<0.05 compared with Ang II+losartan. (B) Cells were pretreated with CC (1 μM) or transfected with AMPK siRNA in the presence of losartan, and then stimulated with Ang II for 48 hrs. Protein expression of p-AMPK was determined by western blot analysis. Representative blots from three independent experiments are shown. |
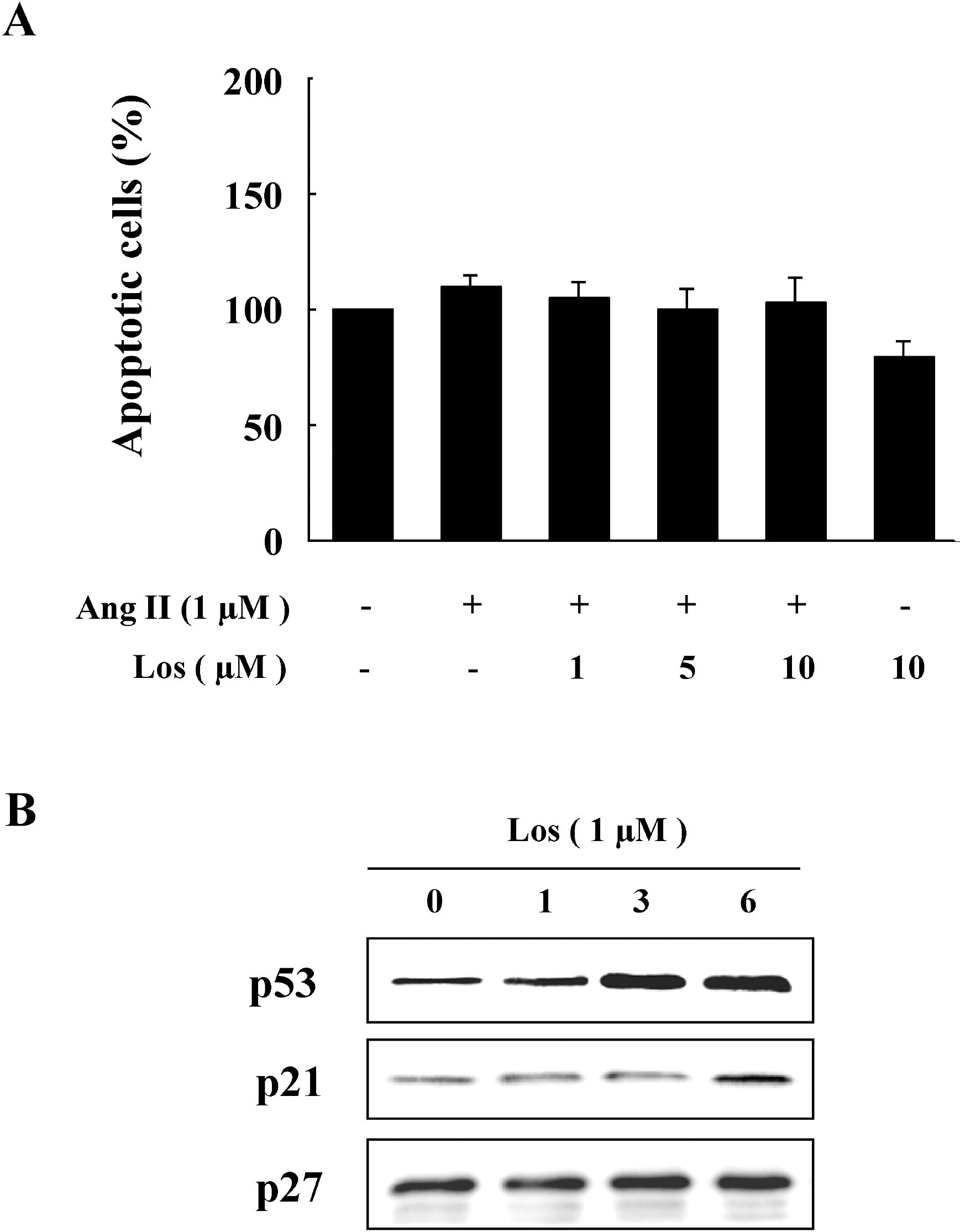 | Fig. 3.Losartan-induced Expressions of p53 and p21. (A) Cells were treated with Ang II in the presence or absence of losartan for 48 hrs. Apoptosis was assessed by Annexin V-fluorescein isothiocyanate (FITC) staining by flow cytometric analysis and the percentage of apoptotic cells was then determined. (B) Cells were treated with losartan for the indicated periods of time. Protein expressions of p53, p21, and p27 were determined by western blot analysis. Representative blots from three independent experiments are shown. |
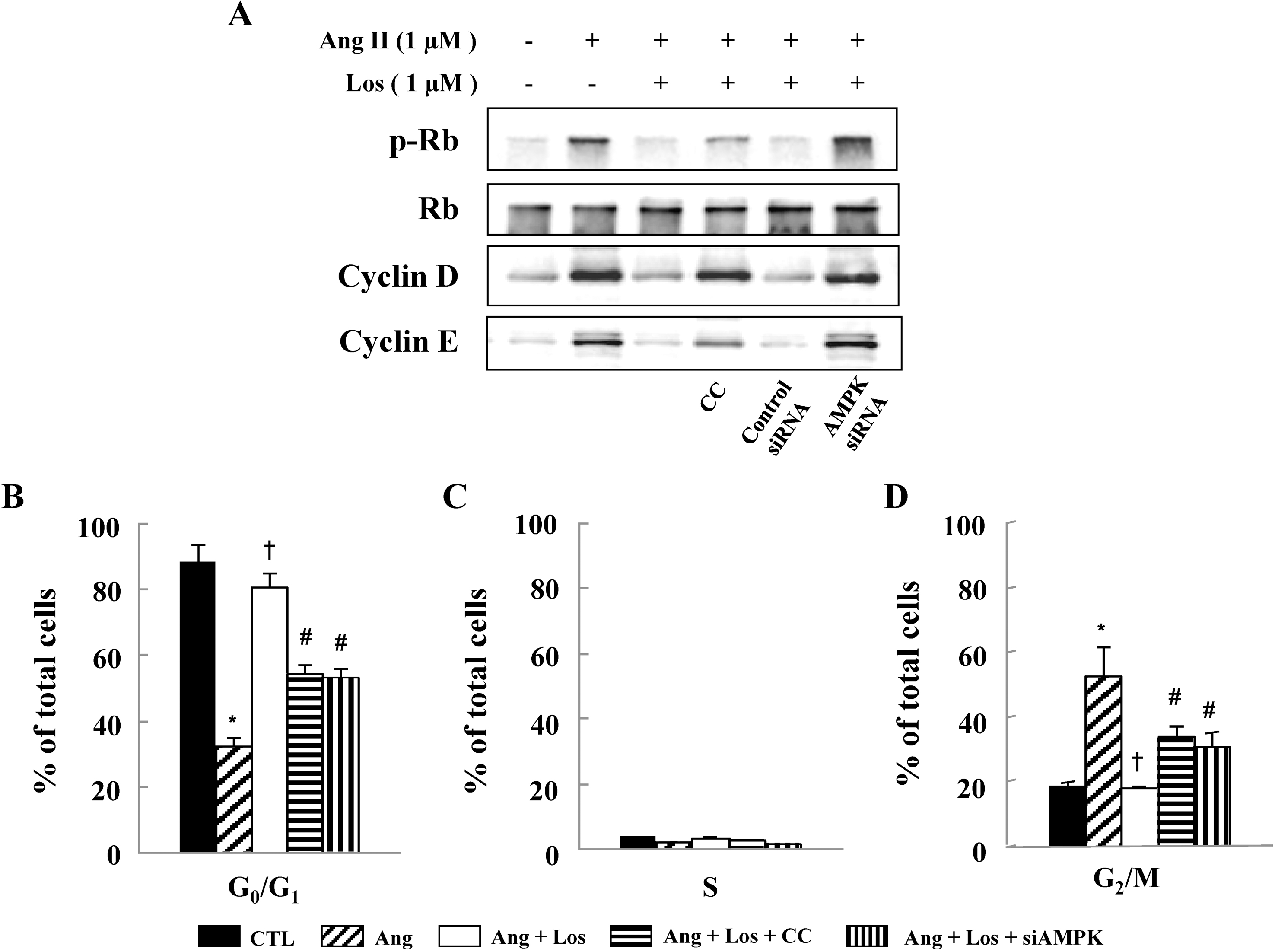 | Fig. 4.Losartan Inhibits Cell Cycle Progression via AMPK activation. Cells were pretreated with compound C (CC) (1 μM) or transfected with control siRNA or AMPK siRNA in the presence of losartan, and then stimulated with Ang II for 24 hrs. (A) Protein expressions of p-Rb, cyclin D, and cyclin E were determined by western blot analysis. Representative blots from three independent experiments are shown. Cell cycle analysis was assessed by PI staining and the percentage of cells in G0/G1 phase (B), S phase (C), and G2/M phase (D). Data are represented as the mean± S.E.M (n=4). ∗p<0.05 compared with control, †p<0.05 compared with Ang II, #p<0.05 compared with Ang II+losartan. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download