Abstract
Urushinol, a plant allergen, has significantly restricted the medical application of Rhus verniciflua, although it has been reported to possess a wide variety of biological activities such as anti-inflammatory, antioxidant, and anti-cancer actions. To reduce the urushinol content while maintaining the beneficial biological activities, mushroom-mediated fermentation of Rhus verniciflua was carried out and this method resulted in significantly attenuated allergenicity [1]. In the present study, to examine the neuroprotective properties of mushroom-fermented stem bark of Rhus verniciflua, two constituents were isolated from mushroom-fermented bark and their neuroprotective properties were examined in a mouse model of kainic acid (KA)-induced excitotoxicity. KA resulted in significant apoptotic neuronal cell death in the CA3 region of mouse hippocampus. However, seven daily administrations of RVH-1 or RVH-2 prior to KA injection significantly attenuated KA-induced pyramidal neuronal cell death in the CA3 region. Furthermore, pretreatment with RVH-1 and RVH-2 also suppressed KA-induced microglial activation in the mouse hippocampus. The present study demonstrates that RVH-1 and RVH-2 isolated from Rhus verniciflua and detoxified using mushroom species possess neuroprotective properties against KA-induced excitotoxicity. This leads to the possibility that detoxified Rhus verniciflua can be a valuable asset in herbal medicine.
Go to : 
References
1. Choi HS. Biological Detoxification of Lacpuer Tree (Rhus verniciflua Stokes) Stem Bark by Mushroom Species. Food Science and Biotechnology. 2007; 16:935–942.
2. Awad AB, Fink CS. Phytosterols as anticancer dietary components: evidence and mechanism of action. J Nutr. 2000; 130:2127–2130.

3. Martins SL, Silva HF, Novaes MR, Ito MK. Therapeutic effects of phytosterols and phytostanols in cholesterolemia. Arch Latinoam Nutr. 2004; 54:257–263.
4. Gabay O, Sanchez C, Salvat C, Chevy F, Breton M, Nourissat G, Wolf C, Jacques C, Berenbaum F. Stigmasterol: a phytosterol with potential anti-osteoarthritic properties. Osteoarthritis Cartilage. 2010; 18:106–116.

5. Chung IM. Cytotoxic Chemical Constituents from the Mushroom of Pholiota adiposa. Food Science and Biotechnology. 2005; 14:255–258.
6. Gupta S. Isolation and characterization of a dihydroxysterol from LAWSONIA INERMIS. Natural Product Letters. 1994; 4:195–201.
7. Greca MD. Stigmasterols from Typha latifolia. J Nat Prod. 1990; 53:1430.
8. Chang YC, Chang FR, Khalil AT, Hsieh PW, Wu YC. Cytotoxic Benzophenanthridine and Benzylisiquinoline Alkaloids from Argemone mexicana. Z Naturforsch C. 2003; 58:521–526.
9. Itokawa H. Several oxidized sterols isolated from callus tissue of Stephania cepharantha. Chem Pharm Bull. 1973; 21:1386–1387.
10. Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990; 13:171–182.

11. Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999; 81:163–221.
12. Izquierdo LA, Barros DM, Ardenghi PG, Pereira P, Rodrigues C, Choi H, Medina JH, Izquierdo I. Different hippocampal molecular requirements for short- and long-term retrieval of one-trial avoidance learning. Behav Brain Res. 2000; 111:93–98.

13. Byun JS, Lee SH, Jeon SH, Kwon YS, Lee HJ, Kim SS, Kim YM, Kim MJ, Chun W. Kainic Acid-induced Neuronal Death is Attenuated by Aminoguanidine but Aggravated by L-NAME in Mouse Hippocampus. Korean J Physiol Pharmacol. 2009; 13:265–271.

14. Giusti P, Lipartiti M, Franceschini D, Schiavo N, Floreani M, Manev H. Neuroprotection by melatonin from kainate-induced excitotoxicity in rats. FASEB J. 1996; 10:891–896.

15. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357.
16. Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993; 52:115–134.

17. Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, Schindler CK, Meller R, Simon RP. Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ. 2001; 8:1169–1181.

18. Badria FA, Dawidar AA, Houssen WE, Shier WT. In vitro study of flavonoids, fatty acids, and steroids on proliferation of rat hepatic stellate cells. Z Naturforsch C. 2005; 60:139–142.

19. Nakai M, Qin ZH, Chen JF, Wang Y, Chase TN. Kainic acid-induced apoptosis in rat striatum is associated with nuclear factor-kappaB activation. J Neurochem. 2000; 74:647–658.
21. Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999; 399:A7–14.

23. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993; 262:689–695.

24. Salinska E, Danysz W, Lazarewicz JW. The role of excitotoxicity in neurodegeneration. Folia Neuropathol. 2005; 43:322–339.
25. Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006; 1088:219–229.

26. Milatovic D, Gupta RC, Dettbarn WD. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002; 957:330–337.

27. Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficincye of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000; 74:2213–2216.
Go to : 
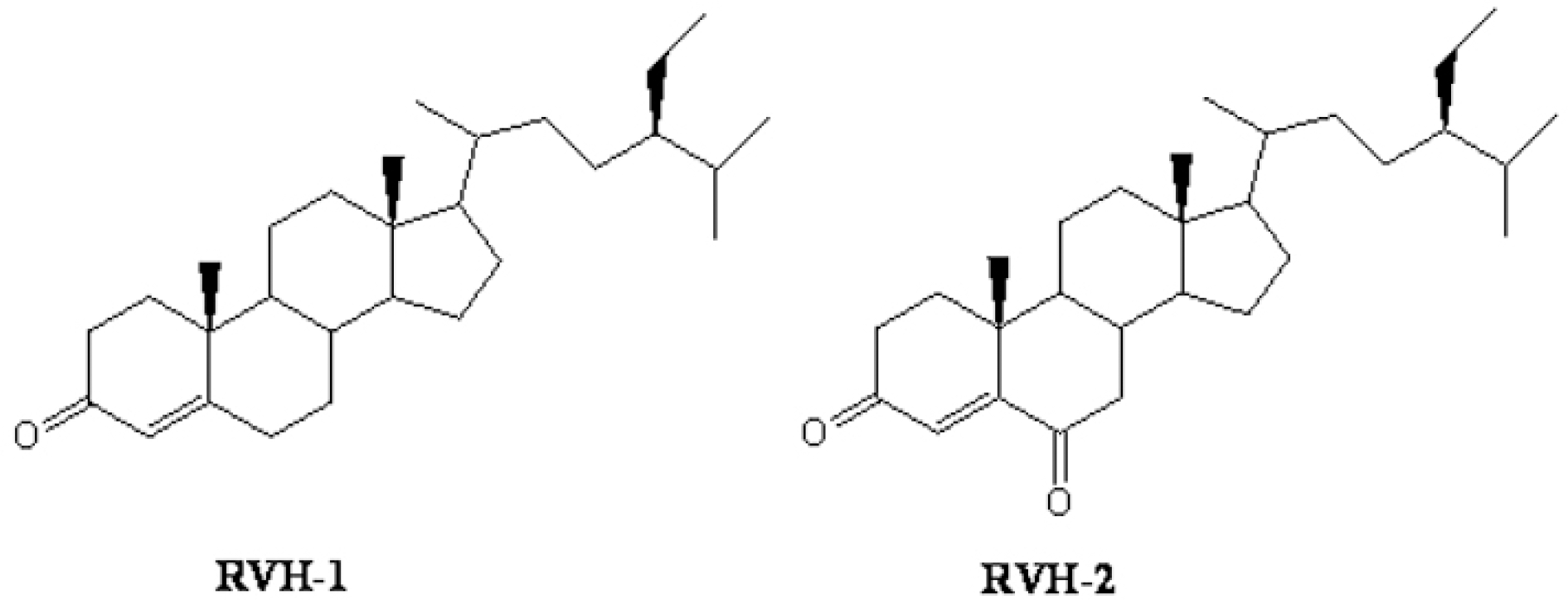 | Fig. 1.Chemical structure of RVH-1 (stigma-4-ene-3-one) and RVH-2 (stigma-4-ene-3,6-dione). |
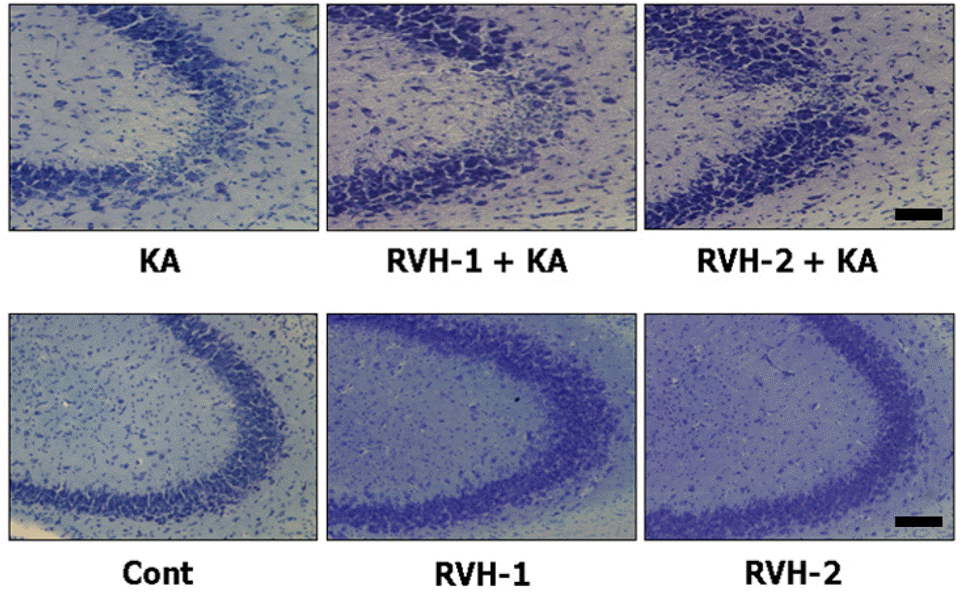 | Fig. 2.Representative neuroprotective effects of RVH-1 and RVH-2 on KA-induced neuronal cell death in CA3 region of mouse hippocampus. Hippocampal cell death was examined with cresyl violet staining. Intracerebroventricular (icv) injection of KA showed marked loss of neurons in the CA3 region of hippocampus (KA). However, RVH-1 or RVH-2 treatment prior to KA injection showed attenuation of neuronal cell loss compared to KA alone. RVH-2 appeared to be more protective than RVH-1, albeit not significantly so. Neuronal cell death was not observed in vehicle (Cont)-, RVH-1-only-, or RVH-2-only-treated mice. More than three mice were used for each group. Scale bar: 100 μm. |
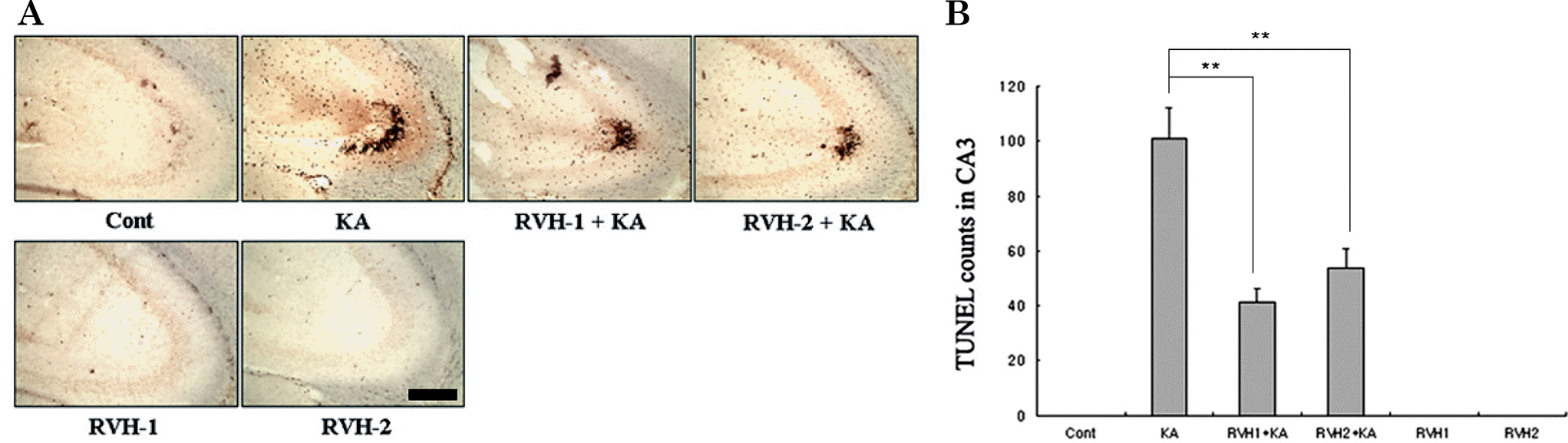 | Fig. 3.Representative (A) and quantitative (B) analysis of neuronal cell death with Terminal deoxytransferase-mediated dUTP-nick end labeling (TUNEL) assay. A considerable number of TUNEL-positive neurons appeared with KA treatment within the CA3 region after 24 hr. Pre-administration of RVH-1 or RVH-2 significantly reduced the number of TUNEL-positive cells compared to the KA-only group. RVH-2 pretreatment showed fewer TUNEL-positive cells than RVH-1. A single treatment of RVH-1 or RVH-2 had no noticeable effects on cell survival. More than three mice were used for each group. Quantitative data represent three independent experiments and are expressed as mean±SEM. ∗∗p<0.01 indicates statistically significant difference from the KA-only treated group. Scale bar: 100 μm. |
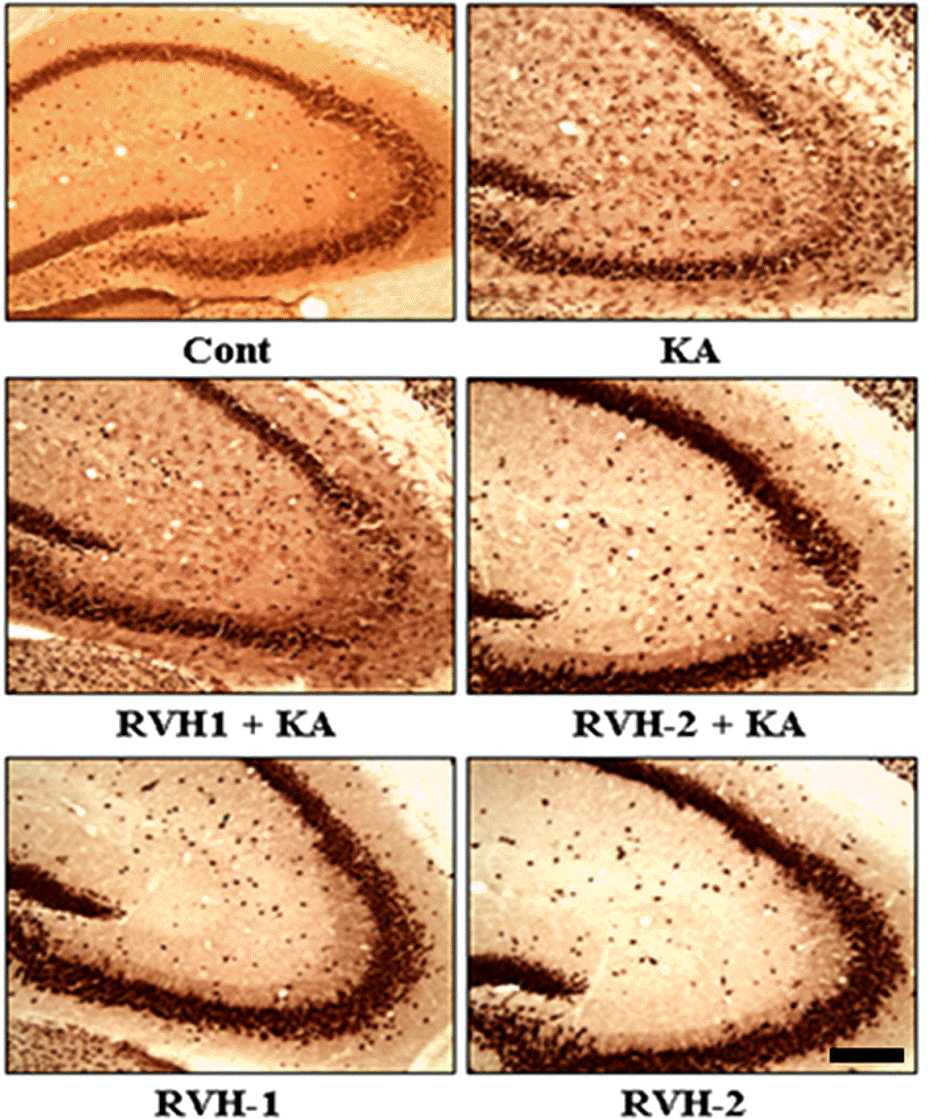 | Fig. 4.Representative images of neuronal protection by RVH-1 and RVH-2 against KA-induced neuronal cell death in the CA3 region of hippocampus. In order to further elucidate the neuroprotective properties of RVH-1 and RVH-2, NeuN immunoreactivity, which specifically stains pyramidal neurons in the hippocampus, was examined. Pretreatment of RVH-1 or RVH-2 attenuated KA-induced death of pyramidal neurons in the CA3 region of hippocampus. RVH-2 showed more neuronal protection against KA-induced excitotoxicity compared to RVH-1. RVH-1 and RVH-2 showed no noticeable change in neuronal viability. More than three mice were used for each group. Scale bar: 100 μm. |
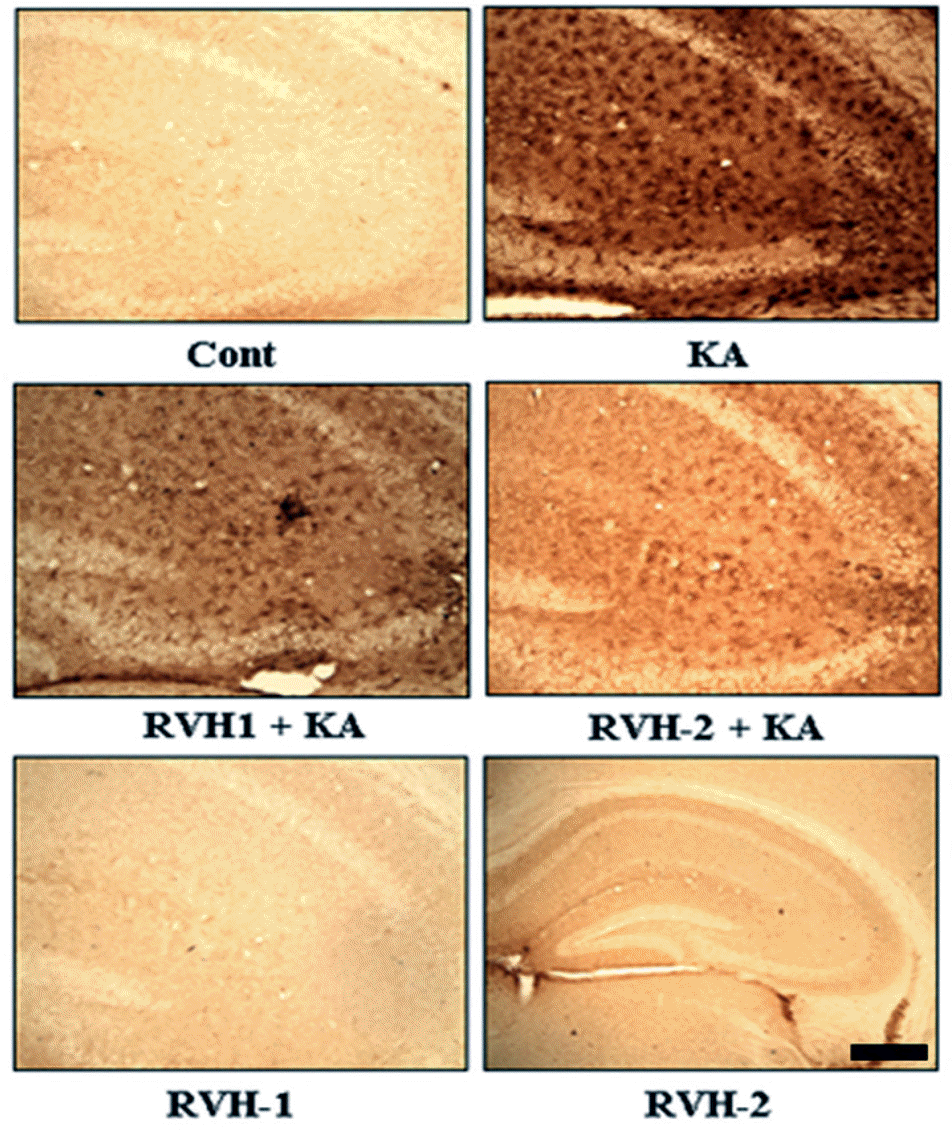 | Fig. 5.Suppressive effects of RVH-1 and RVH-2 on KA-induced microglial activation. Given the previous report that KA-mediated neuronal death accompanies microglial activation, expression of OX-6, a microglial activation marker, was examined with immunohistochemical staining at 24 hr after KA or vehicle treatments. KA resulted in increased microglial activation (KA). However, KA-induced microglial activation was attenuated with RVH-1 or RVH-2 pretreatment. RVH-2 appears to be more suppressive than RVH-1. RVH-1 or RVH-2 showed negligible effects on microglial activation. Representative images were obtained from three independent experiments. More than three mice were used for each group. Scale bar: 100 μm. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download