Abstract
Visnagin (4-methoxy-7-methyl-5H-furo[3,2-g][1]-benzopyran-5-one), which is an active principle extracted from the fruits of Ammi visnaga, has been used as a treatment for low blood-pressure and blocked blood vessel contraction by inhibition of calcium influx into blood cells. However, the neuroprotective effect of visnagin was not clearly known until now. Thus, we investigated whether visnagin has a neuroprotective effect against kainic acid (KA)-induced neuronal cell death. In the cresyl violet staining, pre-treatment or post-treatment visnagin (100 mg/kg, p.o. or i.p.) showed a neuroprotective effect on KA (0.1 μg) toxicity. KA-induced gliosis and proinflammatory marker (IL-1β, TNF-α, IL-6, and COX-2) inductions were also suppressed by visnagin administration. These results suggest that visnagin has a neuroprotective effect in terms of suppressing KA-induced pathogenesis in the brain, and that these neuroprotective effects are associated with its anti-inflammatory effects.
Go to : 
References
3. Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol. 1986; 19:105–111.

4. Jin Y, Lim CM, Kim SW, Park JY, Seo JS, Han PL, Yoon SH, Lee JK. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Research. 2009; 1281:108–116.

5. Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J. Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res. 2005; 79:522–534.

6. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996; 19:312–318.

7. Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997; 20:570–577.

8. Kim JB, Yu YM, Kim SW, Lee JK. Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated efficacious neuroprotection in the postischemic brain. Brain Research. 2005; 1060:188–192.

10. Anrep GV, Barsoum GS, Kenawy MR, Misrahy G. Ammi Visnaga in the treatment of the anginal syndrome. Br Heart J. 1946; 8:171–177.

11. Anrep GV, Barsoum GS, Kenawy MR. The pharmacological actions of the crystalline principles of Ammi Visnaga Linn. J Pharm Pharmacol. 1949; 1:164–176.

12. Duarte J, Perez-Vizcaino F, Torres AI, Zarzuelo A, Jimenez J, Tamargo J. Vasodilator effects of visnagin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1995; 286:115–122.

13. Rauwald HW, Brehm O, Odenthal KP. The involvement of a Ca2+ channel blocking mode of action in the pharmacology of Ammi visnaga fruits. Planta Medica. 1994; 60:101–105.
14. Ubeda A, Tejerina T, Tamargo J, Villar A. Effects of khellin on contractile responses and 45Ca2+ movements in rat isolated aorta. J Pharm Pharmacol. 1991; 43:46–48.
15. Duarte J, Torres AI, Zarzuelo A. Cardiovascular effects of visnagin on rats. Planta Medica. 2000; 66:35–39.
16. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357.
17. Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985; 5:1222–1227.

18. Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed.San Diego: Academic Press;1997.
19. Kwon MS, Seo YJ, Choi SM, Choi HW, Jung JS, Park SH, Suh HW. The differential effects of single or repeated restraint stress on kainic acid-induced neuronal death in the hippocampal CA3 region: the role of glucocorticoid and various signal molecules. J Neurochem. 2007; 103:1530–1541.

20. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987; 162:156–159.

21. Kim SW, Yu YM, Piao CS, Kim JB, Lee JK. Inhibition of delayed induction of p38 mitogen-activated protein kinase attenuates kainic acid-induced neuronal loss in the hippocampus. Brain Research. 2004; 1007:188–191.

22. Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000; 21:395–401.
23. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007; 87:873–904.

24. White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000; 179:1–33.

26. Cho IH, Kim SW, Kim JB, Kim TK, Lee KW, Han PL, Lee JK. Ethyl pyruvate attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J Neurosci Res. 2006; 84:1505–1511.

27. Yoo KY, Hwang IK, Kim JD, Kang IJ, Park J, Yi JS, Kim JK, Bae YS, Won MH. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother Res. 2008; 22:1527–1532.
28. Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002; 295:2282–2285.
29. Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005; 25:3219–3228.
30. Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003; 23:8692–8700.
31. Wang S, Cheng Q, Malik S, Yang J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000; 292:497–504.
32. McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996; 47:425–432.
33. Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997; 17:2746–2755.

34. Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998; 95:10954–10959.

35. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993; 262:689–695.

36. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001; 294:1871–1875.

37. Aboul-Enein HY, Kladna A, Kruk I, Lichszteld K, Michalska T. Effect of psoralens on Fenton-like reaction generating reactive oxygen species. Biopolymers. 2003; 72:59–68.

38. Buttini M, Appel K, Sauter A, Gebicke-Haerter PJ, Boddeke HW. Expression of tumor necrosis factor alpha after focal cerebral ischaemia in the rat. Neuroscience. 1996; 71:1–16.

39. Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992; 149:2736–2741.
40. Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001; 76:846–854.

Go to : 
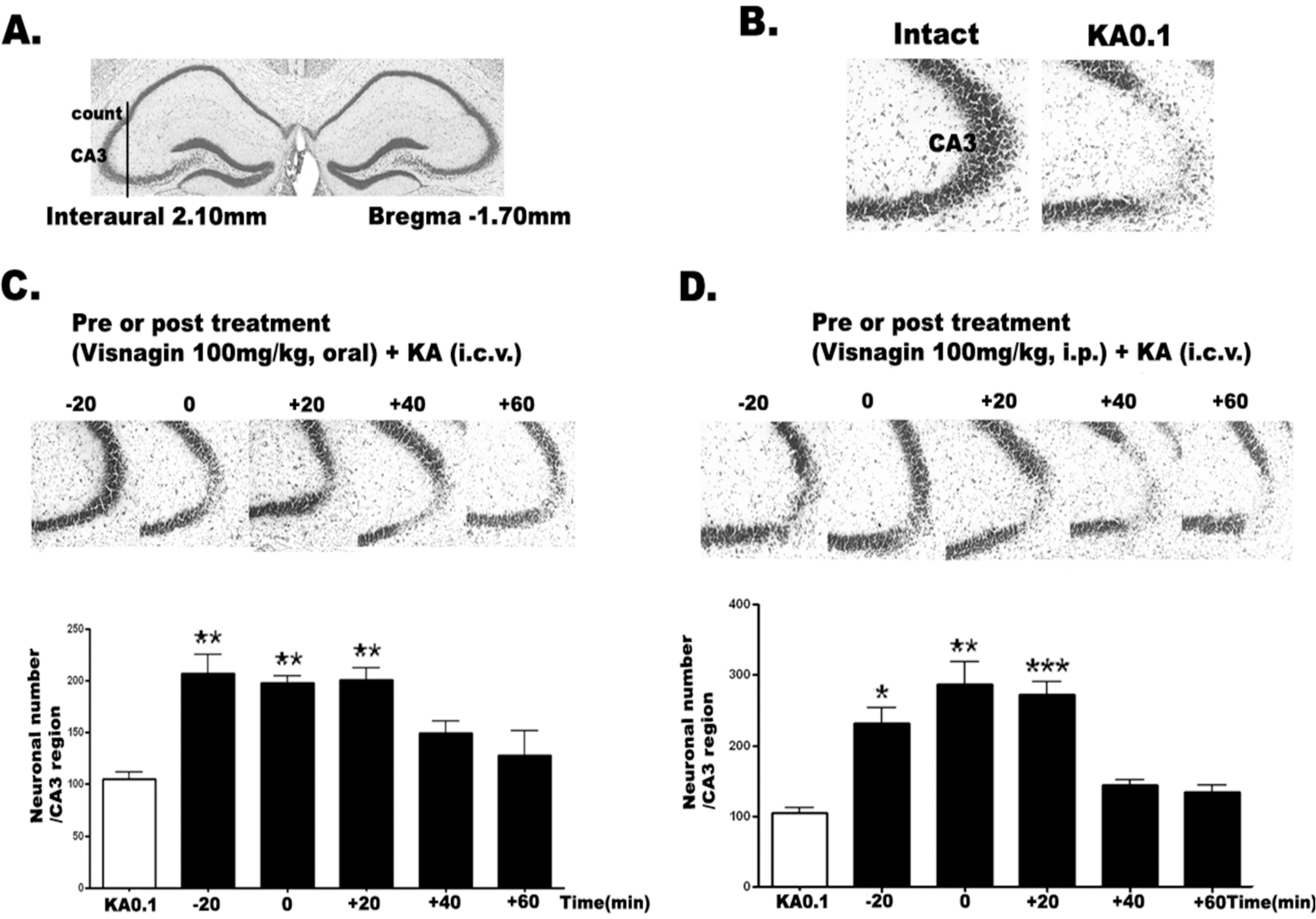 | Fig. 1.The effect of visnagin administered orally or intraperitoneally on KA-induced neuronal death in the hippocampus. The location of the hippocampal CA3 region performing cresyl violet-positive neuronal count is indicated (A) referring Franklin [18]. I.c.v. kainic acid injecton induced neuronal cell death in the pyramidal cells in the CA3 region of hippocampus (B). Mice were administered visnagin orally (C) or intraperitoneally (D) 20 min (– 20) prior to KA (0.1 μg/5 μl) injection or 0, 20 (+ 20), 40 (+40), 60 (+ 60) min after KA treatment (0.1 μg/5 μl). And then, the cresyl violet staining was performed at 1 day after KA. The vertical bars indicate the standard error of mean. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 (KA 0.1 vs other groups). The mice number of each group was 10. |
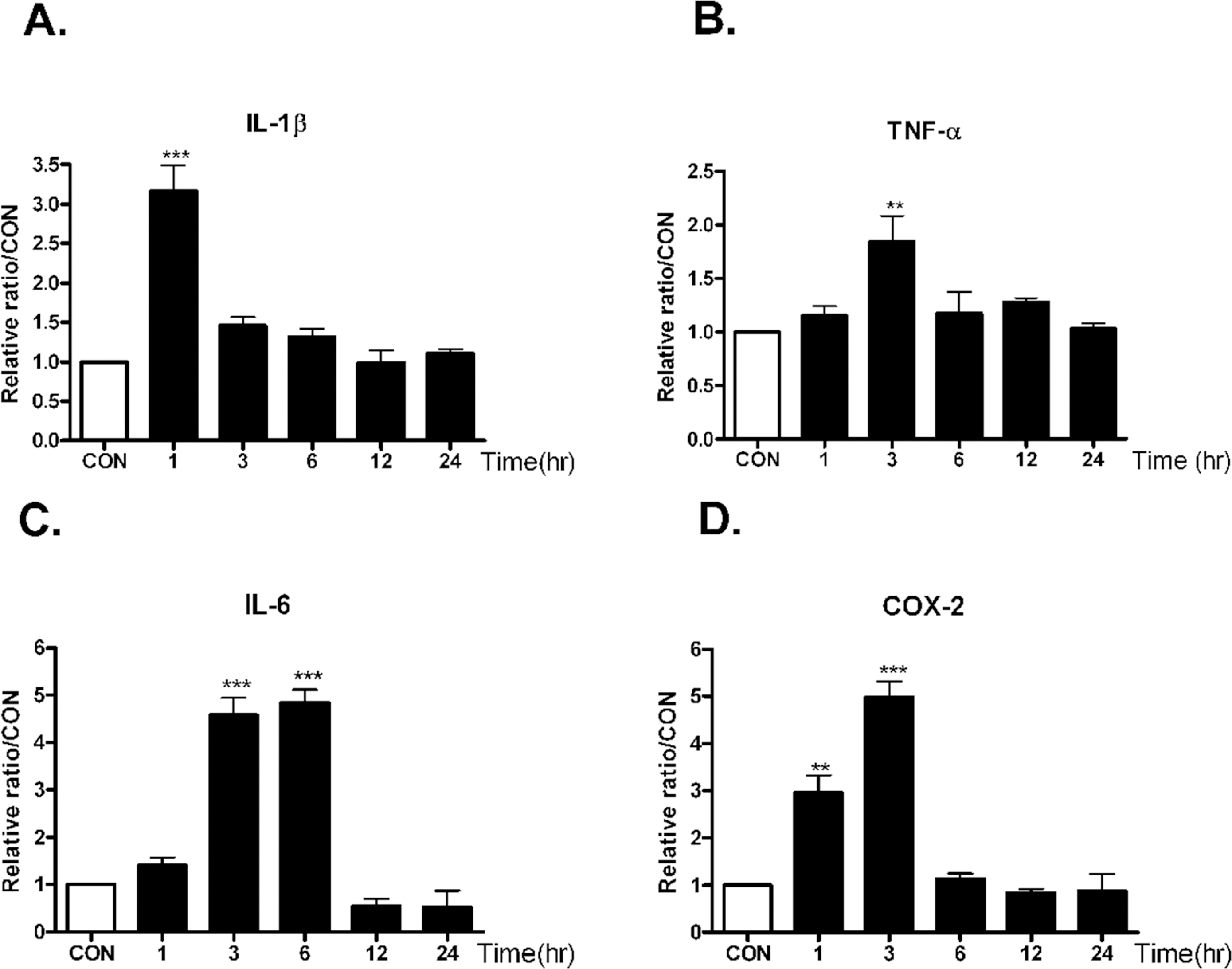 | Fig. 2.The time course alteration of proinflammatory cytokines mRNA on KA injection in the hippocampus. Alteration of IL-1β, TNF-α (B), IL-6 (C), and COX-2 (D) mRNA at 1, 3, 6, 12, 24 hrs after intracerebral (i.c.v.) KA (0.1 μg/5 μl) injectio was examined in the hippocampus. Control group (CON) was injected with i.c.v. PBS. The mice number of each group was 3. Experiments were conducted independently three times, giving a total of nine per group in the final statistical analysis. ∗∗p<0.01(CON vs other groups), ∗∗∗p<0.001 (CON vs other groups). |
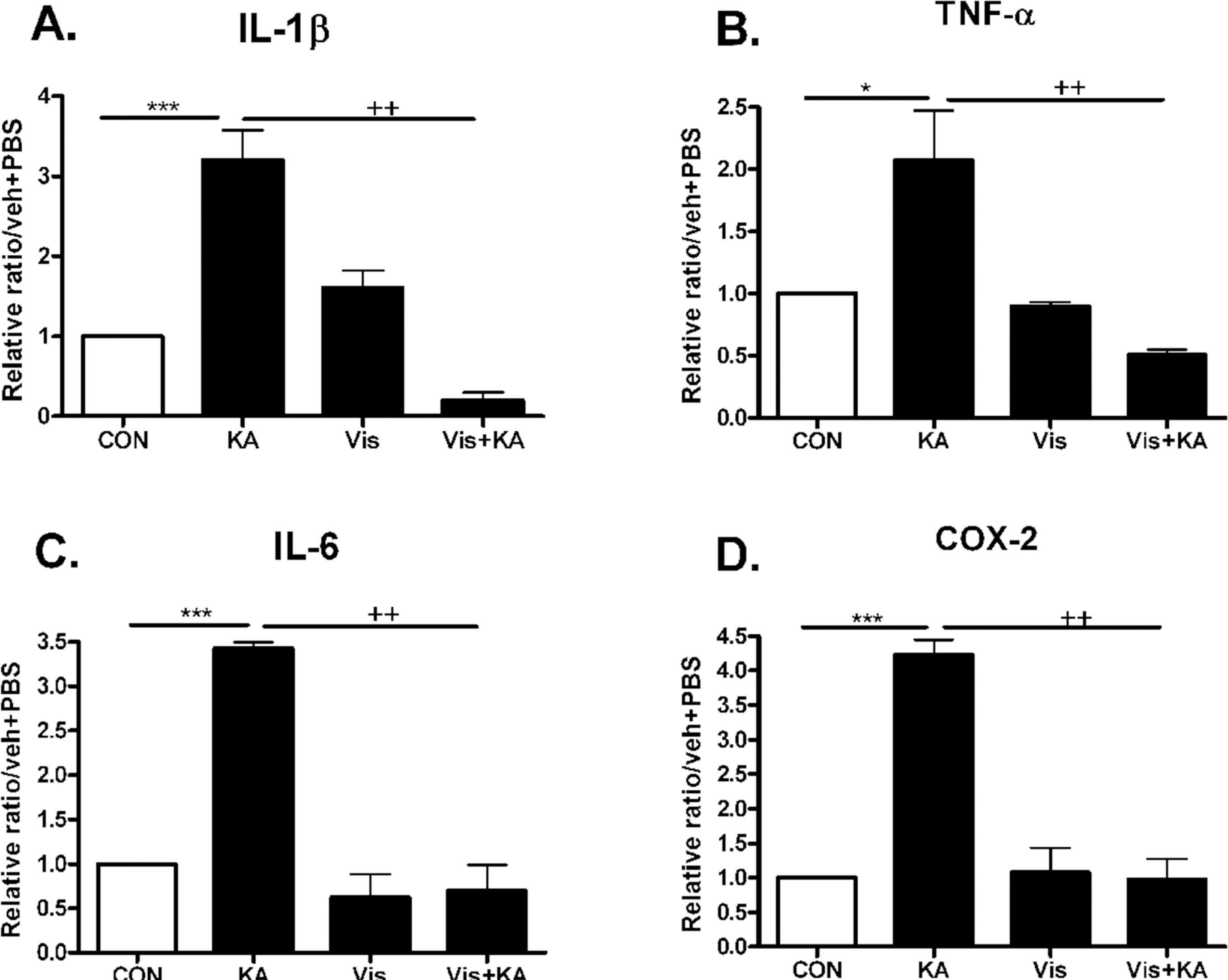 | Fig. 3.The effect of visnagin on proinflammatory cytokines increased by KA in the hippocampus. Alteration of –1β, TNF-α (B), IL-6 (C), and COX-2 (D) at 1 (A), 3 hrs (B, D), or 6 hrs (C) after intracerebroventricular (i.c.v.) KA injection was examined in the hippocampus. The increased IL-1β, TNF-α (B), IL-6 (C), and COX-2 (D) by KA was decreased by visnagin treated prior to 20 min. The mice number of each group was 3. Experiments were conducted independently three times, giving a total of nine per group in the final statistical analysis. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 (CON vs KA), ++p<0.01 (Vis vs Vis + KA). CON, vehicle (i.p.) + PBS (i.c.v.); KA, vehicle (i.p.) + KA (i.c.v.); Vis, Visnagin (i.p.) + PBS (i.c.v.). |
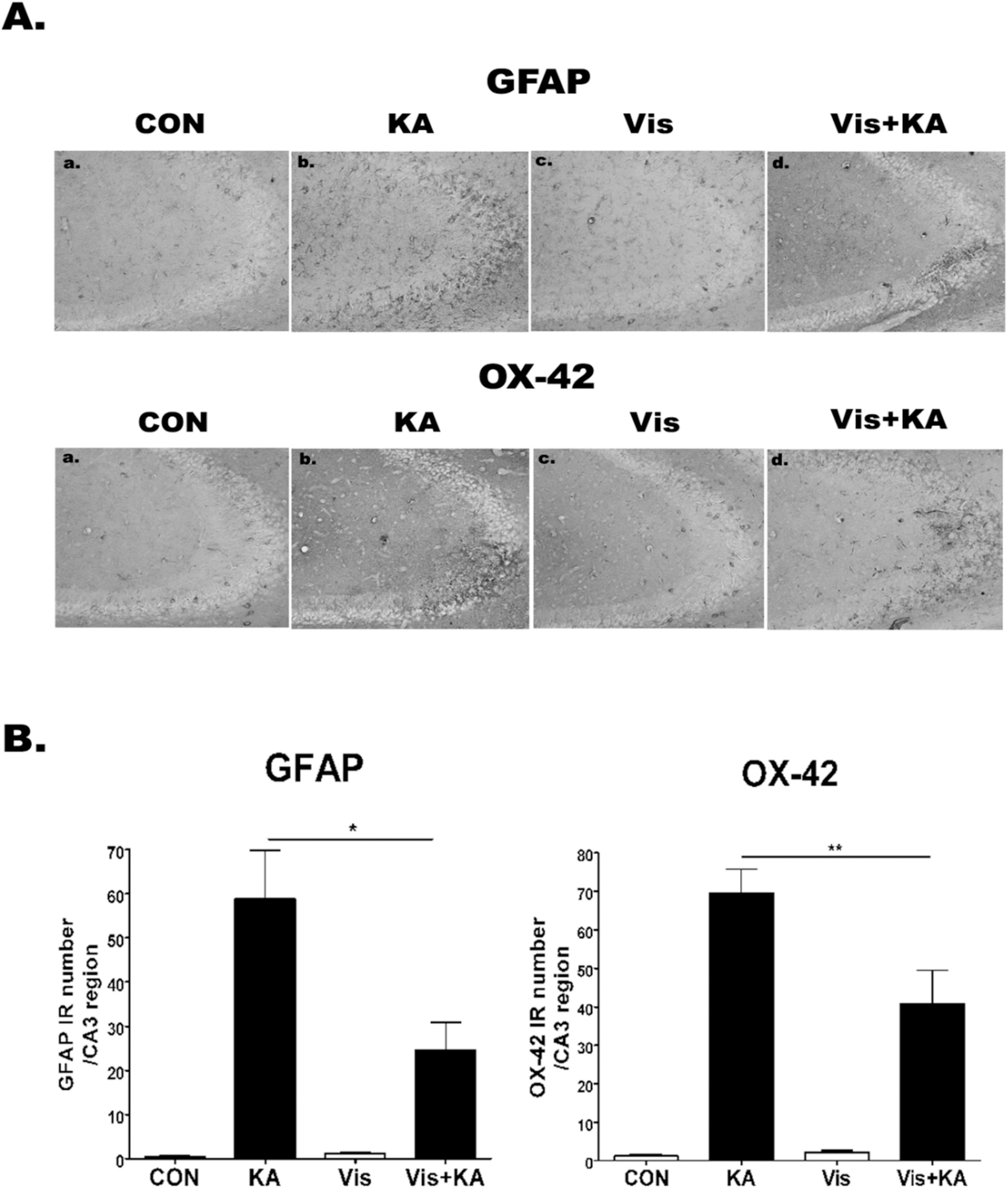 | Fig. 4.The effect of visnagin on the GFAP, OX-42 expression induced by KA in hippocampal CA3 region. OX-42 and GFAP immunoreactivities (A) in the hippocampus were examined at 24 hr after i.c.v. injection of KA (0.1 μg/5 μl). Animals (N=5 per each group) were pretreated orally with either PBS or visnagin (100 mg/kg) 20 min prior to KA (0.1 μg/5 μl). To quantify, we counted GFAP or OX-42 IR in each section (B). CON (a): vehicle (i.p.) + PBS (i.c.v.), KA (b): vehicle (i.p.) + KA (i.c.v.), Vis (c): Visnagin (i.p.) + PBS (i.c.v.), Vis + KA (d): Visnagin (i.p.) + KA (i.c.v.). Antibody against OX-42 and GFAP was used at 1: 10,000 dilution for immunostaining. ∗p<0.05 (KA vs Vis + KA), ∗∗p<0.01 (KA vs Vis + KA). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download