Abstract
Toll-like receptors (TLRs) functionally expressed in salivary epithelial cells, but their roles remain elusive. Among TLRs family, TLR3 is activated by dsRNA, a byproduct of viral infection. The aim of this study was to investigate the role of TLR3 in the inflammatory immune responses using HSG cells. Reverse transcriptase-polymerase chain reaction (RT-PCR), real-time PCR and ELISA were performed to identify expression of TLRs and TLR3-mediated chemokine inductions. The chemotaxis assay of activated T lymphocytes was also performed. Treatment of HSG cells with polyinosinic: polycytidylic acid (poly(I:C)) significantly increased interferon-γ-inducible protein 10 (IP-10), interferon-inducible T-cell α chemoattractant (I-TAC), and regulated on activation, normal T-cells expressed and secreted (RANTES) gene expressions in a concentration-dependent manner. Anti-TLR3 antibody blocked the increases of IP-10 and I-TAC genes. Poly(I:C)-induced increases of IP-10 and I-TAC were also confirmed at protein levels from cell lysates, but their release into extracellular medium was detected only in IP-10. We found that the culture media from HSG cells stimulated with poly(I:C) significantly increases T lymphocyte migration. Our results suggest that TLR3 plays an important role in chemokine induction, particularly IP-10, in salivary epithelial cells.
Go to : 
References
2. Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Kasama T, Adachi M. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-kappaB and/or IRF-3 in airway epithelial cells. Clin Exp Allergy. 2006; 36:1049–1062.
3. Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, Wuyts A, Opdenakker G, Van Damme J. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol. 2003; 33:3146–3153.
4. Ritter M, Mennerich D, Weith A, Seither P. Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. J Inflamm (Lond). 2005; 2:16.

5. Greene CM, McElvaney NG. Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz). 2005; 53:418–427.
6. Schaefer TM, Fahey JV, Wright JA, Wira CR. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly (I:C). Am J Reprod Immunol. 2005; 54:193–202.

7. Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994; 152:5532–5539.
8. Ramos-Casals M, Munoz S, Medina F, Jara LJ, Rosas J, Calvo-Alen J, Brito-Zeron P, Forns X, Sanchez-Tapias JM. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009; 36:1442–1448.

9. Prunoiu C, Georgescu EF, Georgescu M, Simionescu C. Sjogren's syndrome associated with chronic hepatitis C – the benefit of the antiviral treatment. Rom J Morphol Embryol. 2008; 49:557–562.
10. Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006; 53:248–256.

11. Ohyama Y, Carroll VA, Deshmukh U, Gaskin F, Brown MG, Fu SM. Severe focal sialadenitis and dacryoadenitis in NZM2328 mice induced by MCMV: a novel model for human Sjogren's syndrome. J Immunol. 2006; 177:7391–7397.
12. Kawakami A, Nakashima K, Tamai M, Nakamura H, Iwanaga N, Fujikawa K, Aramaki T, Arima K, Iwamoto N, Ichinose K, Kamachi M, Ida H, Origuchi T, Eguchi K. Toll-like receptor in salivary glands from patients with Sjogren's syndrome: functional analysis by human salivary gland cell line. J Rheumatol. 2007; 34:1019–1026.
13. Spachidou MP, Bourazopoulou E, Maratheftis CI, Kapsogeorgou EK, Moutsopoulos HM, Tzioufas AG, Manoussakis MN. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren's syndrome. Clin Exp Immunol. 2007; 147:497–503.
14. Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999; 162:3549–3558.
15. Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998; 101:746–754.

16. Doyle S, Vaidya S, O'Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002; 17:251–263.

17. Doyle SE, O'Connell R, Vaidya SA, Chow EK, Yee K, Cheng G. Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4. J Immunol. 2003; 170:3565–3571.

18. Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998; 187:2009–2021.

19. Deshmukh US, Nandula SR, Thimmalapura PR, Scindia YM, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009; 38:42–7.

20. Szodoray P, Alex P, Brun JG, Centola M, Jonsson R. Circulating cytokines in primary Sjogren's syndrome determined by a multiplex cytokine array system. Scand J Immunol. 2004; 59:592–599.

21. Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren's syndrome. Arthritis Rheum. 2002; 46:2730–2741.
22. Zhou W, Dong Y, Zhao Y, Tang FL. Abnormal interferon-inducible protein-10 expression in the labial glands of patients with Sjogren's syndrome. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003; 25:603–607.
23. Ogawa N, Shimoyama K, Kawanami T. Molecular mechanisms of salivary gland destruction in patients with Sjogren's syndrome. Nihon Rinsho Meneki Gakkai Kaishi. 2005; 28:10–20.
24. Kasman LM, London LL, London SD, Pilgrim MJ. A mouse model linking viral hepatitis and salivary gland dysfunction. Oral Dis. 2009; 15:587–595.

25. Hasegawa H, Inoue A, Kohno M, Muraoka M, Miyazaki T, Terada M, Nakayama T, Yoshie O, Nose M, Yasukawa M. Antagonist of interferon-inducible protein 10/CXCL10 ameliorates the progression of autoimmune sialadenitis in MRL/lpr mice. Arthritis Rheum. 2006; 54:1174–1183.

26. Kim SK, Kim YM, Yeum CE, Jin SH, Chae GT, Lee SB. Rifampicin Inhibits the LPS-induced Expression of Toll-like Receptor 2 via the Suppression of NF-kappaB DNA-binding Activity in RAW 264.7 Cells. Korean J Physiol Pharmacol. 2009; 13:475–482.
Go to : 
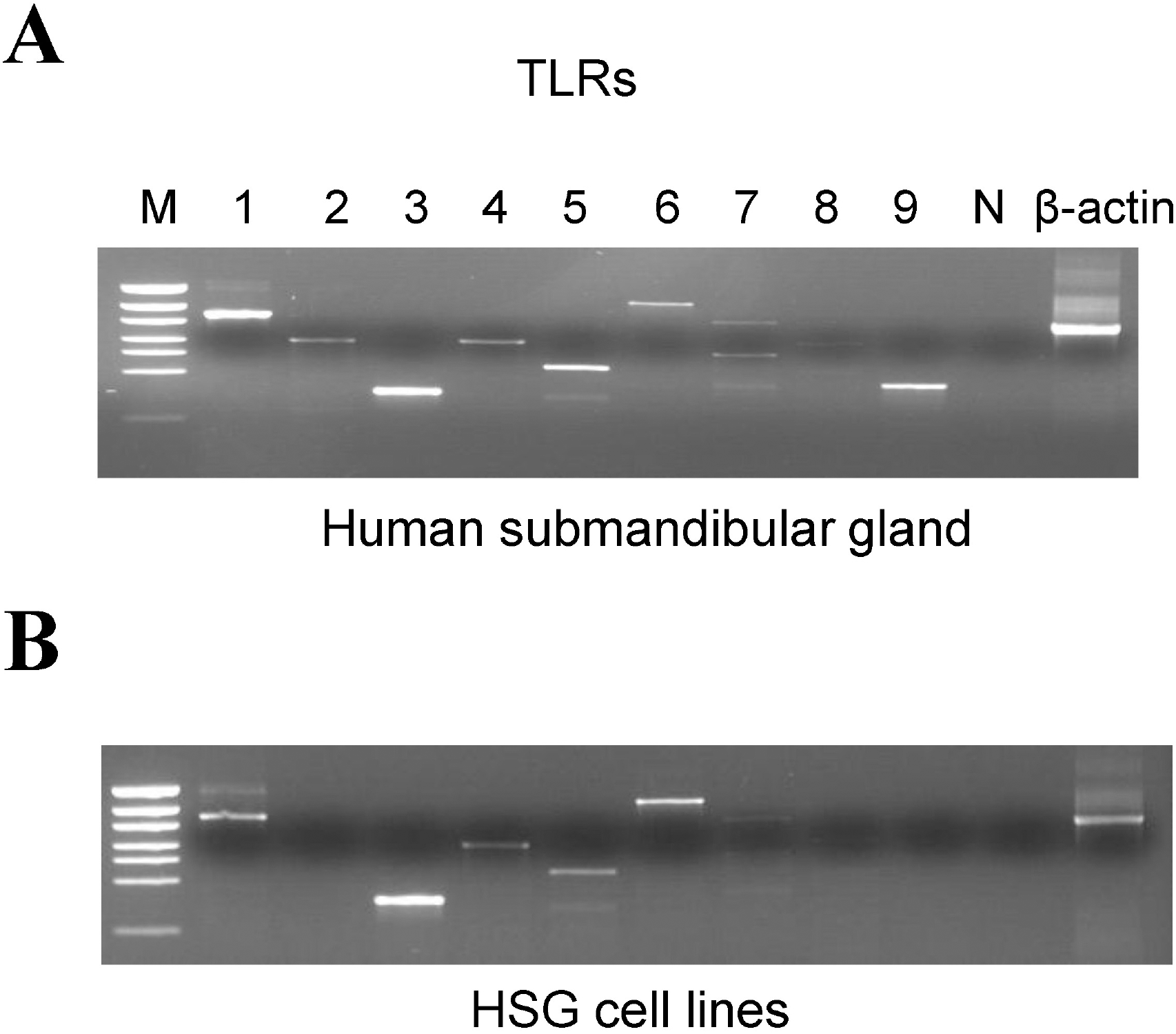 | Fig. 1.mRNA expression of Toll like receptor (TLR) subtypes in human submandibular glands (hSMG) and HSG cell lines. (A) Strong mRNA expression of TLR1, 3, 5, and 9 and weak expression of TLR2, 4, and 6 in hSMG. M; marker protein, Pwon600DNA/ECOR1+HinfI Digest N; negative control. (B) Strong mRNA expression of TLR1, 3 and 6, and weak expressions TLR4 and 5 in HSG cell lines. |
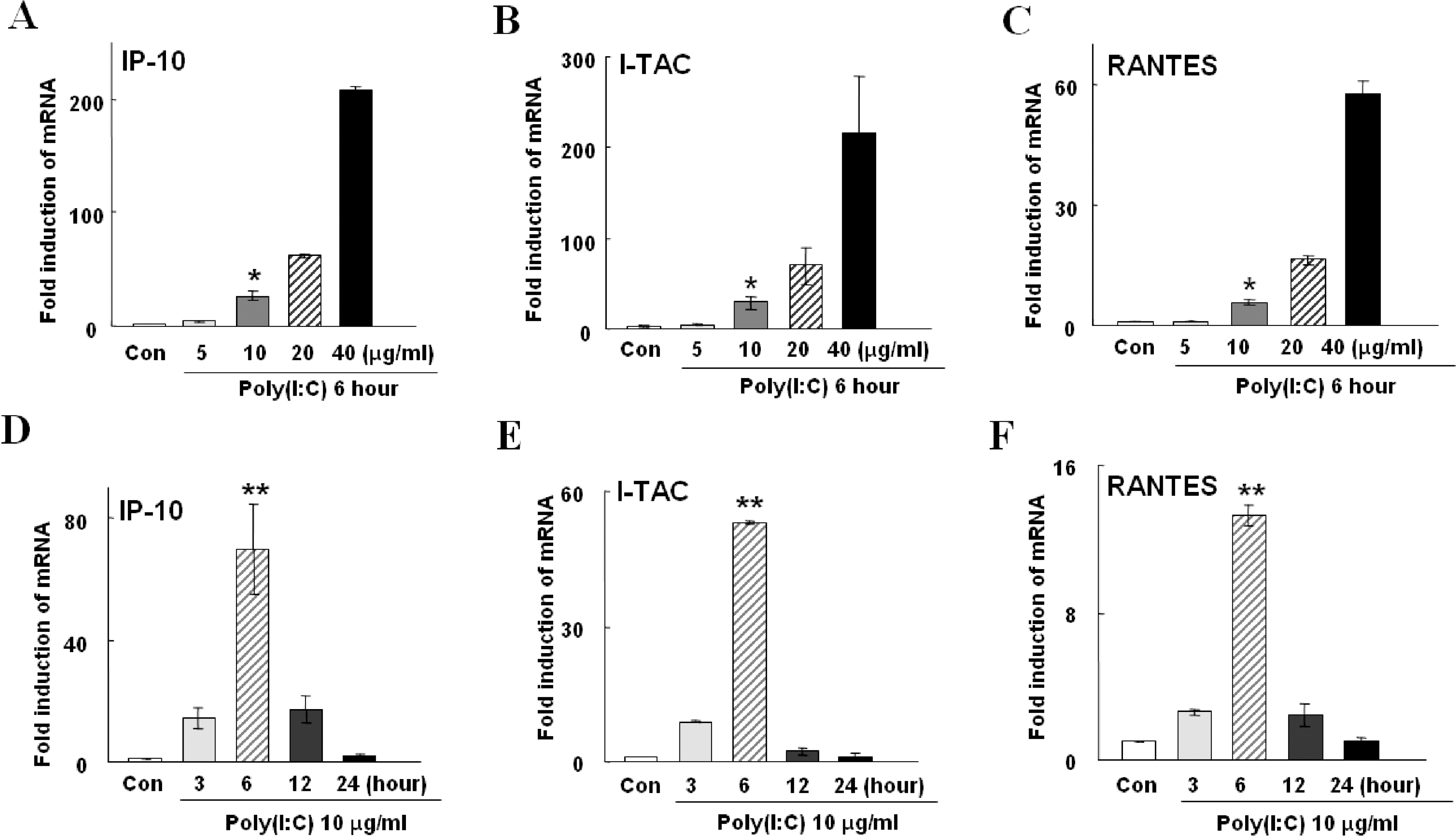 | Fig. 2.Poly(I:C)-induced mRNA expressions of IP-10, I-TAC, and RANTES in HSG cells. The means±S.E.M of three independent experiments are shown. (A∼C) Increase in chemokine gene expression in a concentration-dependent manner. Treatment of cells with 10 μg/ml poly(I:C) significantly increased (p<0.01, indicated by ∗) induction of IP-10, I-TAC, and RANTES by 26.5±1.2, 28.6±10.0, and 5.6±0.9 folds (dark grey bar in A, B, C), respectively, compared to the control (Con). The high concentration of poly(I:C), 40 μg/ml, further increased inductions of these chemokines by 209.3±19.7, 216.2±88.3, and 57.6±4.7 folds, respectively (black bar in A, B, C). (D∼F) Expression levels of chemokine genes in a various incubation time with 10 μg/ml of poly(I:C). Peak increases of all three chemokine expressions were observed after incubation of cells with poly(I:C) for 6 hrs by 68.3±2.3, 53±0.6, and 13.2±0.7 folds (p<0.001, indicated by ∗∗), respectively (hatched bar in D, E, and F). |
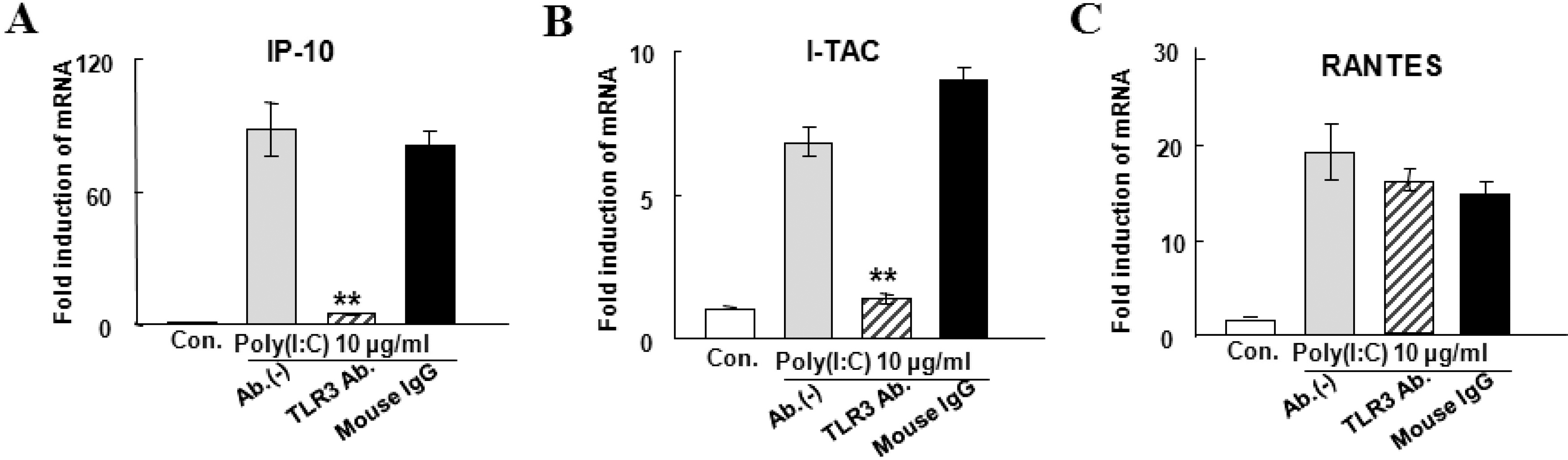 | Fig. 3.Effects of TLR3 antibody on the IP-10 (A), I-TAC (B), and RANTES (C) mRNA expression induced by 10 μg/ml poly(I:C) for 6 hrs. The means±S.E.M of three independent experiments are shown. Poly(I:C)-induced inductions of IP-10, I-TAC and RANTES mRNA were decreased to 4.7±0.09, 1.4±0.3, and 14±5 folds, respectively, by the addition of 20 μg/ml TLR3 antibody (hatched bars). TLR3 antibody significantly decreased IP-10 and I-TAC mRNA expression (p<0.001, indicated by ∗∗), but not RANTES. Addition of mouse IgG (black bars) instead of TLR3 antibody has no effect on the expression of the three chemokine genes. |
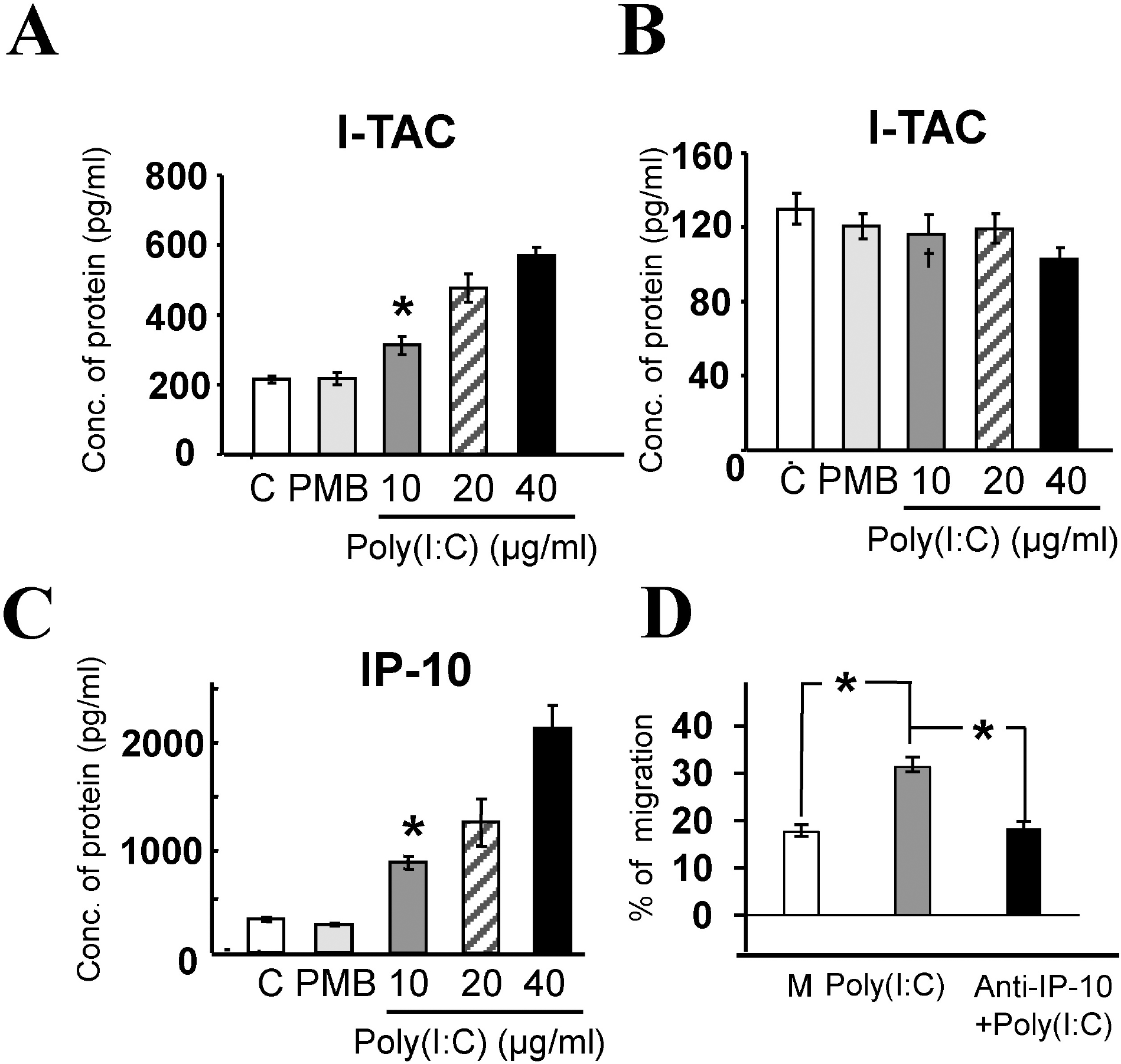 | Fig. 4.Poly(I:C)-induced chemokine release and migration of activated T lymphocytes. The means±S.E.M of three independent experiments are shown. (A) I-TAC protein concentration from cell lysates in the control (C, 215±9.2 pg/ml), PMB (217.7±16.7 pg/ml), and poly(I:C) treatment groups. 10 μg/ml of poly(I:C) significantly increased protein concentration to 312.6±26.4 pg/ml (p<0.01, indicated by ∗), compared to the control. (B) I-TAC protein concentrations in culture medium in control (C), PMB, and poly(I:C) treatment groups with 10, 20, and 40 μg/ml concentrations. The amount of I-TAC protein in the poly(I:C) treatment group was not significantly different, compared to the control or PMB groups (p> 0.1). Note the different scale of Y-axis. (C) IP-10 protein concentrations in control (313.1±20.1 pg/ml), PMB (262.6±13.1 pg/ml), and poly(I:C) treatment groups in culture media. 10 μg/ml of poly(I:C) significantly increased protein concentration to 837.4±58.7 pg/ml (p< 0.001, indicated by ∗). (D) % migration of activated T lymphocytes induced by three different incubation media: untreated (control, white bar, 17.6±1.5%, n=3), treated with 10 μg/ml poly(I:C) (grey bar, 31.3±2.0%, n=3), or plus antibodies against IP-10 (black bar, 18.3±1.5%, n=3). 10 μg/ml poly(I:C) significantly increased migration of activated T lymphocytes compared to the control (p<0.01, indicated by ∗), and addition of anti-IP-10 Ab completely blocked the poly(I:C)-induced T lymphocyte migration. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download