Abstract
Endometritis is one of the primary reasons for reproductive failure. In order to investigate endometritis-associated marker proteins, proteomic analysis was performed on bovine endometrium with endometritis. In bovine endometritis, desmin, α-actin-2, heat-shock protein (HSP) 27, peroxiredoxin-6, luteinizing hormone receptor isoform 1, collectin-43 precursor, deoxyribonuclease-I (DNase-I), and MHC class I heavy chain (MHC-Ih) were up-regulated. In contrast, transferrin, interleukin-2 precursor, hemoglobin β subunit, and potassium channel tetramerisation domain-containing 11 (KCTD11) were down-regulated in comparison to normal endometrium. The proteomic results were validated by semiquantitative-PCR and immunoblot analysis. The mRNA levels of desmin, transferrin, α-actin-2, HSP27, KCTD11, and MHC-Ih were up-regulated by over 1.5-fold, and showed a pattern similar to their proteomic profiles. Desmin and α-actin-2 protein showed positive correlations between proteomic analysis and immunoblot analysis. These results suggest that desmin and α-actin-2 may play important roles in endometritis-related function, and could be useful markers for the diagnosis of bovine endometritis.
References
1. Sheldon IM, Dobson H. Reproductive challenges facing the cattle industry at the beginning of the 21st century. Reprod Suppl. 2003; 61:1–13.

2. Fischer C, Drillich M, Gabler C, Heuwieser W, Einspanier R. Postpartum reproductive failure in cattle: is the examination of gene expression in the endometrium a key to success? Berl Munch Tierarztl Wochenschr. 2006; 119:197–202.
3. McDougall S, Macaulay R, Compton C. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim Reprod Sci. 2007; 99:9–23.

4. Lenz M, Drillich M, Heuwieser W. Evaluation of the diagnosis of subclinical endometritis in dairy cattle using ultrasound. Berl Munch Tierarztl Wochenschr. 2007; 120:237–244.
5. Williams EJ, Fischer DP, Pfeiffer DU, England GC, Noakes DE, Dobson H, Sheldon IM. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. 2005; 63:102–117.

6. Berendt FJ, Frohlich T, Schmidt SE, Reichenbach HD, Wolf E, Arnold GJ. Holistic differential analysis of embryo-induced alterations in the proteome of bovine endometrium in the preattachment period. Proteomics. 2005; 5:2551–2560.

7. Janue A, Odena MA, Oliveira E, Olive M, Ferrer I. Desmin is oxidized and nitrated in affected muscles in myotilinopathies and desminopathies. J Neuropathol Exp Neurol. 2007; 66:711–723.
8. Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991; 87:847–858.

9. Gorman AM, Szegezdi E, Quigney DJ, Samali A. Hsp27 inhibits 6-hydroxydopamine-induced cytochrome c release and apoptosis in PC12 cells. Biochem Biophys Res Commun. 2005; 327:801–810.

10. Ma X, Fan L, Meng Y, Hou Z, Mao YD, Wang W, Ding W, Liu JY. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007; 13:527–535.

11. Davies CJ, Fisher PJ, Schlafer DH. Temporal and regional regulation of major histocompatibility complex class I expression at the bovine uterine/placental interface. Placenta. 2000; 21:194–202.

12. Joyce MM, Burghardt JR, Burghardt RC, Hooper RN, Bazer FW, Johnson GAS. Uterine MHC class I molecules and beta2-microglobulin are regulated by progesterone and conceptus interferons during pig pregnancy. J Immunol. 2008; 181:2494–2505.
13. Prasad S, Soldatenkov VA, Srinivasarao G, Dritschilo A. Intermediate filament proteins during carcinogenesis and apoptosis (Review). Int J Oncol. 1999; 14:563–570.

14. Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004; 301:1–7.

15. Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002; 7:167–176.

16. Maravei DV, Trbovich AM, Perez GI, Tilly KI, Banach D, Talanian RV, Wong WW, Tilly JL. Cleavage of cytoskeletal proteins by caspases during ovarian cell death: evidence that cell-free systems do not always mimic apoptotic events in intact cells. Cell Death Differ. 1997; 4:707–712.

17. Ogburn KD, Figueiredo-Pereira ME. Cytoskeleton/endoplasmic reticulum collapse induced by prostaglandin J2 parallels centrosomal deposition of ubiquitinated protein aggregates. J Biol Chem. 2006; 281:23274–23284.

18. Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008; 12:743–761.

20. Stolpen AH, Pober JS, Brown CS, Golan DE. Class I major histocompatibility complex proteins diffuse isotropically on immune interferon-activated endothelial cells despite anisotropic cell shape and cytoskeletal organization: application of fluorescence photobleaching recovery with an elliptical beam. Proc Natl Acad Sci U S A. 1988; 85:1844–1848.

21. Wu X, Englund K, Lindblom B, Blanck A. mRNA-expression of often used house-keeping genes and the relation between RNA and DNA are sex steroid-dependent parameters in human myometrium and fibroids. Gynecol Obstet Invest. 2003; 55:225–230.

22. Spencer TE, Johnson GA, Bazer FW, Burghardt RC, Palmarini M. Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev. 2007; 19:65–78.

23. Ciocca DR, Asch RH, Adams DJ, McGuire WL. Evidence for modulation of a 24K protein in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1983; 57:496–499.

Fig. 1.
Two-dimensional gel electrophoresis of bovine endometrium (A, B) Representative two-dimensional gel electrophoresis profiles of endometrium obtained from Korean cattle with and without endometritis. The soluble protein (100 μg) of each isolate was loaded on the 2-DE gel. The 2-DE gels were focused on 17-cm IPG strips with immobilized pH ranging 3 to 10 (A) or 5 to 8 (B). Proteins were then separated on 12.5% polyacrylamide gel. Molecular size markers are shown on the left-hand side of the gels. The proteins were visualized by silver staining. Number (1 to 14) represents DEP identified by PDQuest.
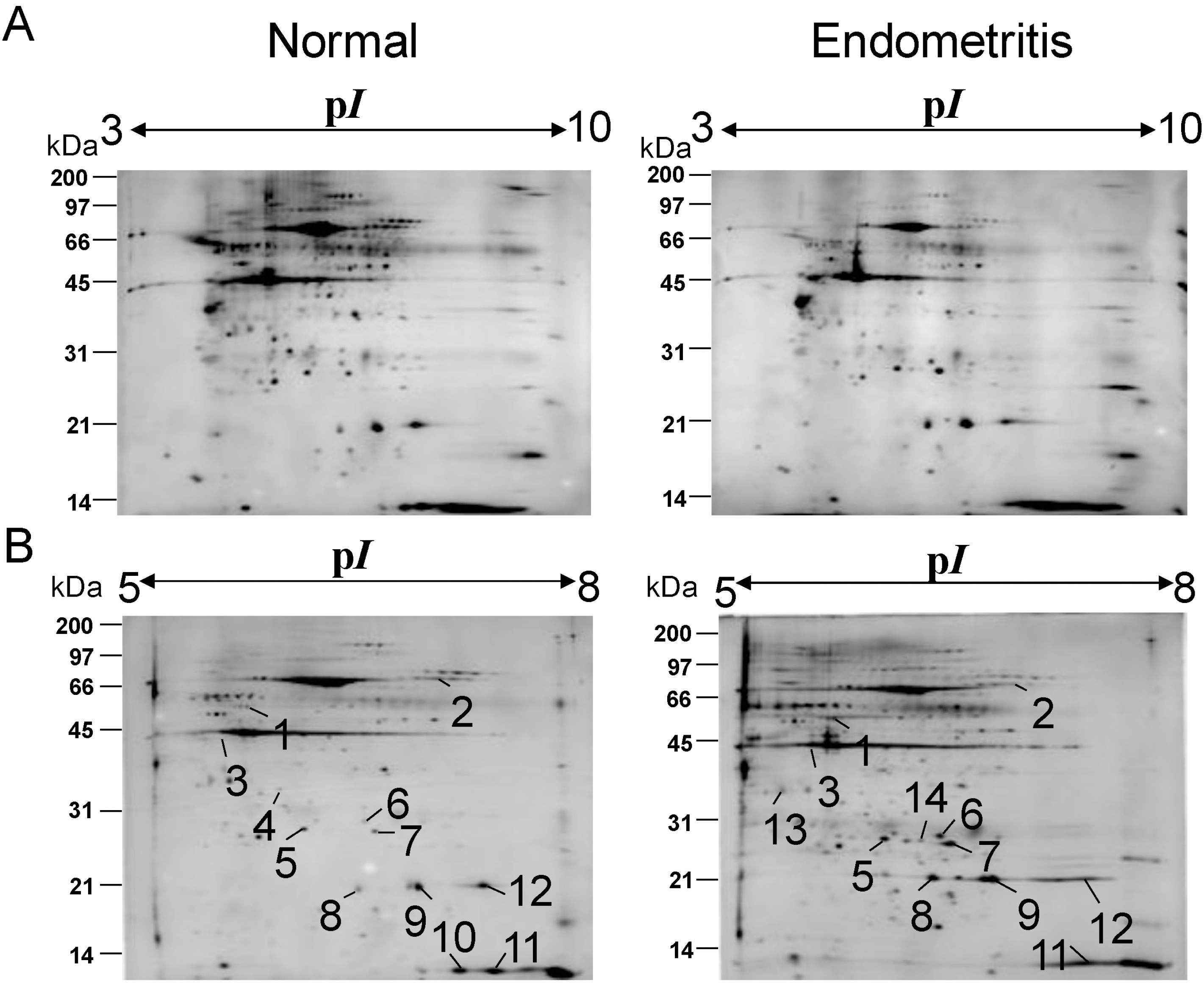
Fig. 2.
Differentially expressed proteins in bovine endometrium with endometritis (A) Details of 2-DE gel images showing selected DEPs. The enlarged images of DEPs contain some additional protein spots. (B) Summary of intensities of DEP spots. Each bar represents mean±SD of four repeated experiments. The asterisks indicate the significant difference from the corresponding control value obtained for normal endometrium (p<0.05).
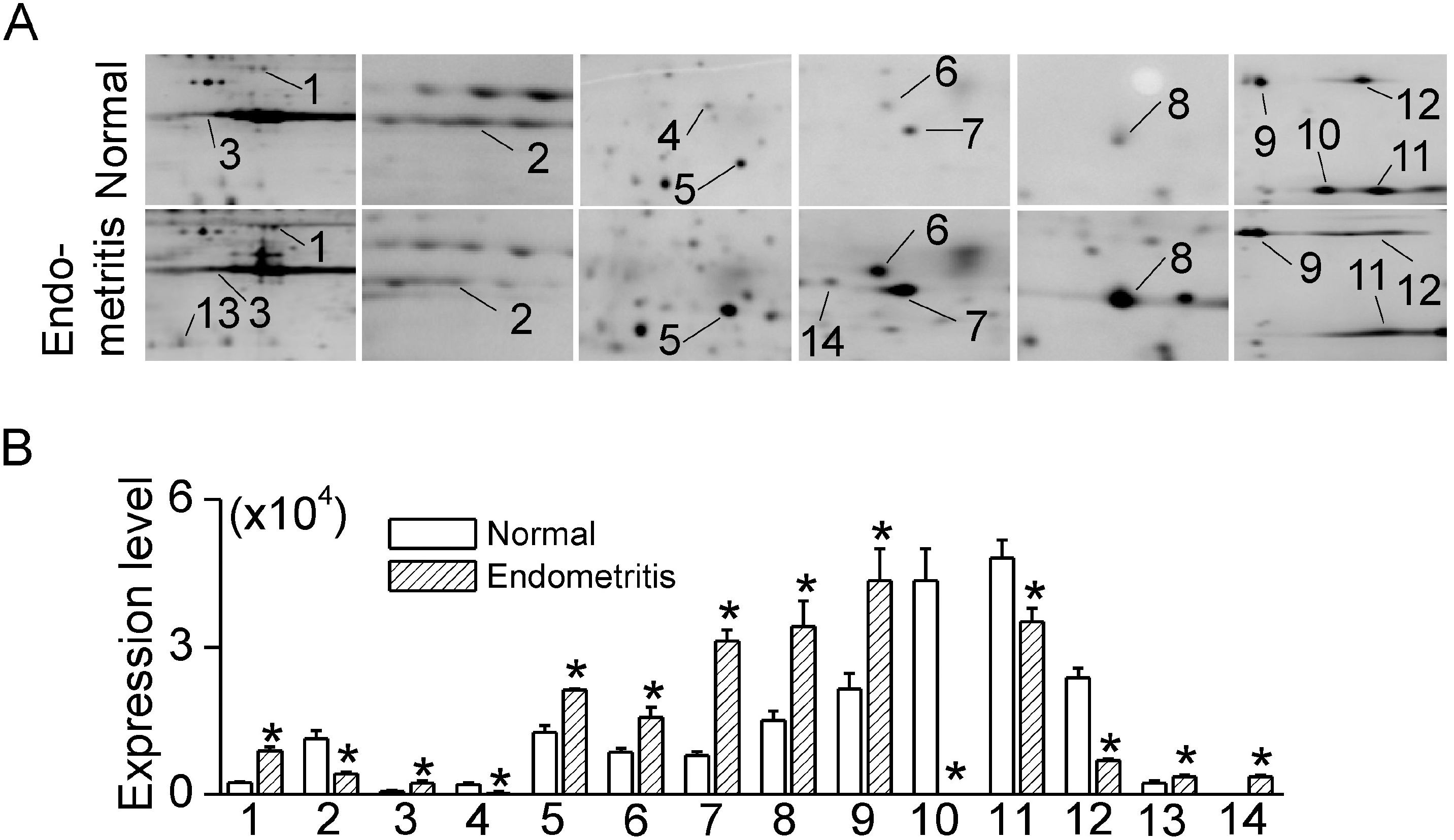
Fig. 3.
Validation of 2-DE analysis by semi-quantitative PCR and western blot analyses in bovine endometrium, with and without endometritis. (A) RT-PCR products for desmin (453-bp), transferring (488-bp), α-actin (329-bp), HSP27 (300-bp), PRSIN-6 (402-bp), KCTD11 (344-bp), and MHC-I (428-bp) derived from bovine endometrium. The first lanes show the 1-kb DNA ladder. Bar graph shows normalized mRNA levels of desmin, transferrin, α-actin, HSP27, PRSIN-6, KCTD11, and MHC-I in the endometria with and without endometritis. The expression levels were normalized to GAPDH. Each bar represents mean±SD of five repeated experiments. The asterisks indicate the significant difference from the corresponding control value obtained for normal endometrium (p<0.05). N and E represent normal endometrium and endometrium with endometritis, respectively. (B, C) Western blot analyses of desmin, α-actin, HSP27 and HSP70 proteins. Ponceau S staining of total proteins served as a loading control. The bar graph shows the up-regulation of desmin and α-actin in endometrium with endometritis. Each bar represents the mean±SD of three repeated experiments. Asterisks indicate the significant difference from the corresponding control value (normal), p<0.05.
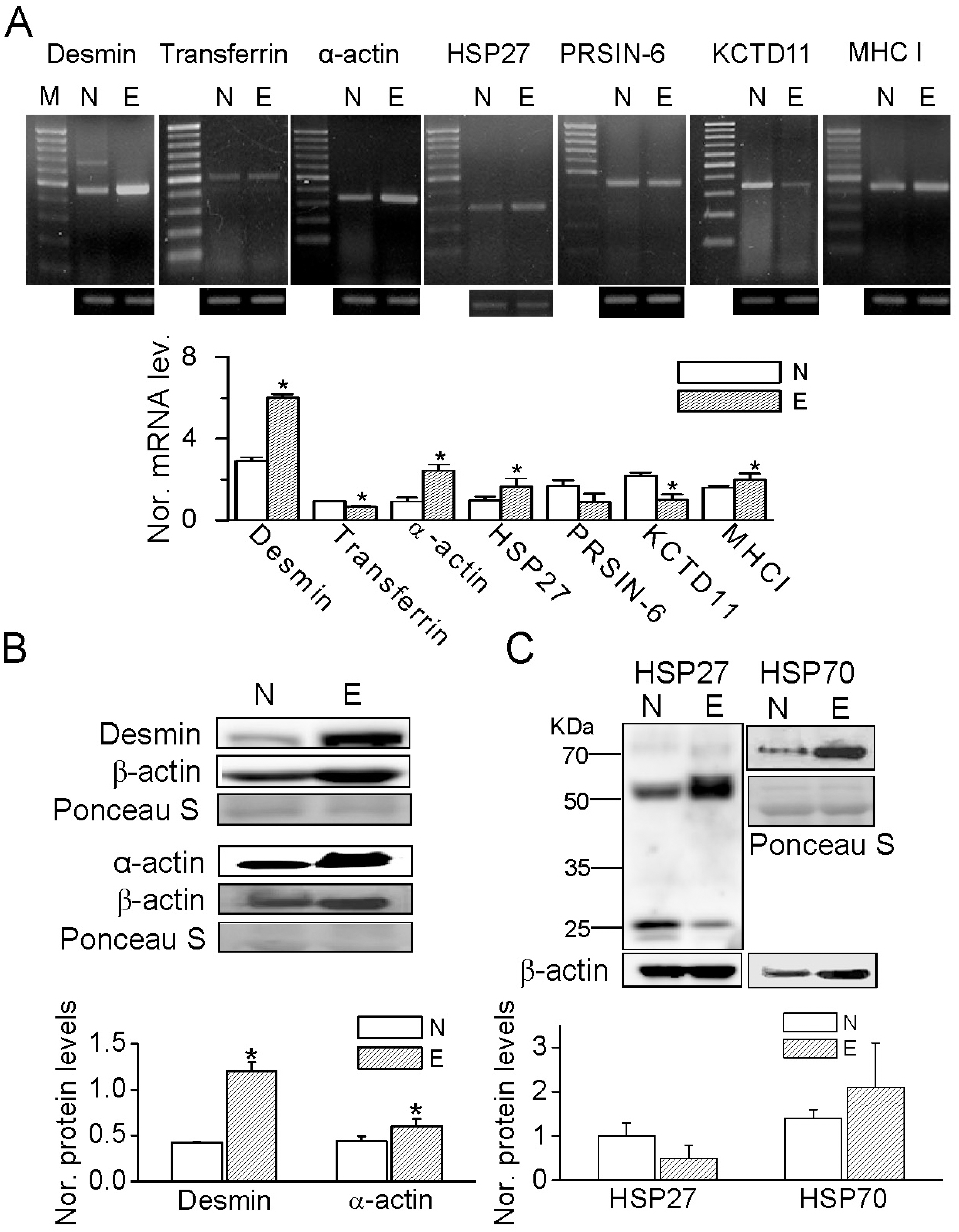
Table 1.
Primer sequences used for RT-PCR
Table 2.
List of differentially expressed proteins identified by MALDI-TOF-MS in the endometrium with endometritis
| Spot No. | Annotation | MW /pI | Swiss-Prot. Acc. Nos. | CV (%) | Fold change | MOWSE score | % Cov |
|---|---|---|---|---|---|---|---|
| 1 | Desmin | 53.4/5.2 | O62654 | 24.8/33.9 | 3.7 (Δ) | 558,976 | 43.7 |
| 2 | Serotransferrin precursor (Transferrin) | 77.8/6.8 | Q29443 | 58.9/37.9 | 0.37 (∇) | 387 | 21.7 |
| 3 | Actin, aortic smooth muscle (α-actin-2) | 42.0/5.2 | P62739 | 0/47.9 | 1.53 (Δ) | 10,758 | 42.2 |
| 4 | Interleukin-2 precursor (IL-2) | 17.6/6.1 | P05016 | – (∇)∗ | 228 | 18.1 | |
| 5 | Heat-shock protein β-1 (HSP27) | 22.4/6.0 | Q3T149 | 26.7/31.4 | 1.68 (Δ) | 123,678 | 45.9 |
| 6 | Peroxiredoxin-6 | 24.9/6.0 | O77834 | 43.4/23.9 | 1.82 (Δ) | 269 | 26.9 |
| 7 | Heat-shock protein beta-1 (HSP27) | 22.4/6.0 | Q3T149 | 10.8/8.2 | 3.93 (Δ) | 35,712 | 44.8 |
| 8 | Luteinizing hormone receptor isoform 1 (Fragment) | 24.7/5.2 | Q28026 | 43.2/22.6 | 2.27 (Δ) | 931 | 36.8 |
| 9 | Collectin-43 precursor (CL-43) | 33.6/5.0 | P42916 | 28.9/12.2 | 2.3 (Δ) | 194 | 28.4 |
| 10 | Hemoglobin β subunit | 16.0/7.0 | P02070 | 10.4/0 | - (∇)∗ | 226 | 51.7 |
| 11 | Hemoglobin β subunit | 16.0/7.0 | P02070 | 0.73 (∇) | 159 | 44.1 | |
| 12 | Potassium channel tetramerisation domain containing 11 (KCTD11) | 20.5/6.4 | Q58DF7 | 0.71 (∇) | 822 | 36.3 | |
| 13 | Deoxyribonuclease-1 | 31.3/5.3 | P00639 | + (Δ)† | 222 | 21.7 | |
| 14 | MHC class I heavy chain (Fragment) | 40.4/5.4 | O78186 | 0/56.4 | + (Δ)† | 23,348 | 42.5 |
Table 3.
List of hypothetical proteins identified by MALDI-TOF-MS in the endometrium with endometritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download