Abstract
The present study demonstrates the effect of fibrates, agonists of PPARα on cytokines-induced proliferation in primary cultured astrocytes. Alone or combination treatment with cytokines, such as IL-1β (10 ng/ml), IFNγ (10 ng/ml), and TNF-α (10 ng/ml) cause a significant increase of cell proliferation in a time-dependent manner. Treatment of astrocytes with bezafibrate and fenofibrate (0, 5, and 10 μM) reduced the IFNγ and IL-1β-induced cell proliferation in a dose-dependent manner. To address the involvement of IL-6 on the IFNγ and IL-β-induced cell proliferation, released IL-6 level was measured. IFNγ and IL-1β cause an increase of released IL-6 protein level in a time-dependent manner. Furthermore, pretreatment with IL-6 antibody (0, 0.1, 1, 2.5, and 5 ng/ml) dose-dependently inhibited the IFNγ and IL-1β-induced cell proliferation. However, bezafibrate and fenofibrate did not affect increased mRNA and protein levels of IL-6 in IFNγ and IL-1β-stimulated astrocytes. Taken together, these results clearly suggest that activation of PPARα attenuates the IFNγ and IL-1β-induced cell proliferation through IL-6 independent pathway.
Go to : 
References
1. Chvatal A, Anderova M, Neprasova H, Prajerova I, Benesova J, Butenko O, Verkhratsky A. Pathological potential of astroglia. Physiol Res. 2008; 57 Suppl. 3:S101–110.
2. Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer's disease. Cell Death Differ. 2009; 16:378–385.
3. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999; 20:649–688.

4. Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004; 4:61–70.

5. Lalloyer F, Staels B. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2010; 30:894–899.

7. Pannu R, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of tumor necrosis factor alpha-mediated proliferation of rat primary astrocytes. Implications for astrogliosis following neurotrauma. J Biol Chem. 2005; 280:13742–13751.
8. Gao Q, Li Y, Shen L, Zhang J, Zheng X, Qu R, Liu Z, Chopp M. Bone marrow stromal cells reduce ischemia-induced astrocytic activation in vitro. Neuroscience. 2008; 152:646–655.

9. Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990; 144:129–135.
10. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997; 94:4312–4317.
11. Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007; 178:1904–1913.
12. Sun GJ, Xie XM, Xing Y, Yan WH, Yang TL, Yu GL. Effects of fenofibrate on the proliferation and apoptosis and nitric oxide synthase expression of cultured human umbilical vein endothelial cells induced by lysophosphatidylcholine. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006; 31:373–378.
13. Gizard F, Amant C, Barbier O, Bellosta S, Robillard R, Percevault F, Sevestre H, Krimpenfort P, Corsini A, Rochette J, Glineur C, Fruchart JC, Torpier G, Staels B. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest. 2005; 115:3228–3238.
14. Perez-Ortiz JM, Tranque P, Vaquero CF, Domingo B, Molina F, Calvo S, Jordan J, Cena V, Llopis J. Glitazones differentially regulate primary astrocyte and glioma cell survival. Involvement of reactive oxygen species and peroxisome proliferator-activated receptor-gamma. J Biol Chem. 2004; 279:8976–8985.
15. Ogata T, Miyauchi T, Irukayama-Tomobe Y, Takanashi M, Goto K, Yamaguchi I. The peroxisome proliferator-activated receptor alpha activator fenofibrate inhibits endothelin-1-induced cardiac fibroblast proliferation. J Cardiovasc Pharmacol. 2004; 44 Suppl. 1:S279–282.
16. Pogue AI, Cui JG, Li YY, Zhao Y, Culicchia F, Lukiw WJ. Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett. 2010; 476:18–22.

17. Quintana A, Molinero A, Borup R, Nielsen FC, Campbell IL, Penkowa M, Hidalgo J. Effect of astrocyte-targeted production of IL-6 on traumatic brain injury and its impact on the cortical transcriptome. Dev Neurobiol. 2008; 68:195–208.

18. Cardenas H, Bolin LM. Compromised reactive microgliosis in MPTP-lesioned IL-6 KO mice. Brain Res. 2003; 985:89–97.

19. Penkowa M, Giralt M, Carrasco J, Hadberg H, Hidalgo J. Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia. 2000; 32:271–285.

20. Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J Neuroimmunol. 2006; 176:95–105.
Go to : 
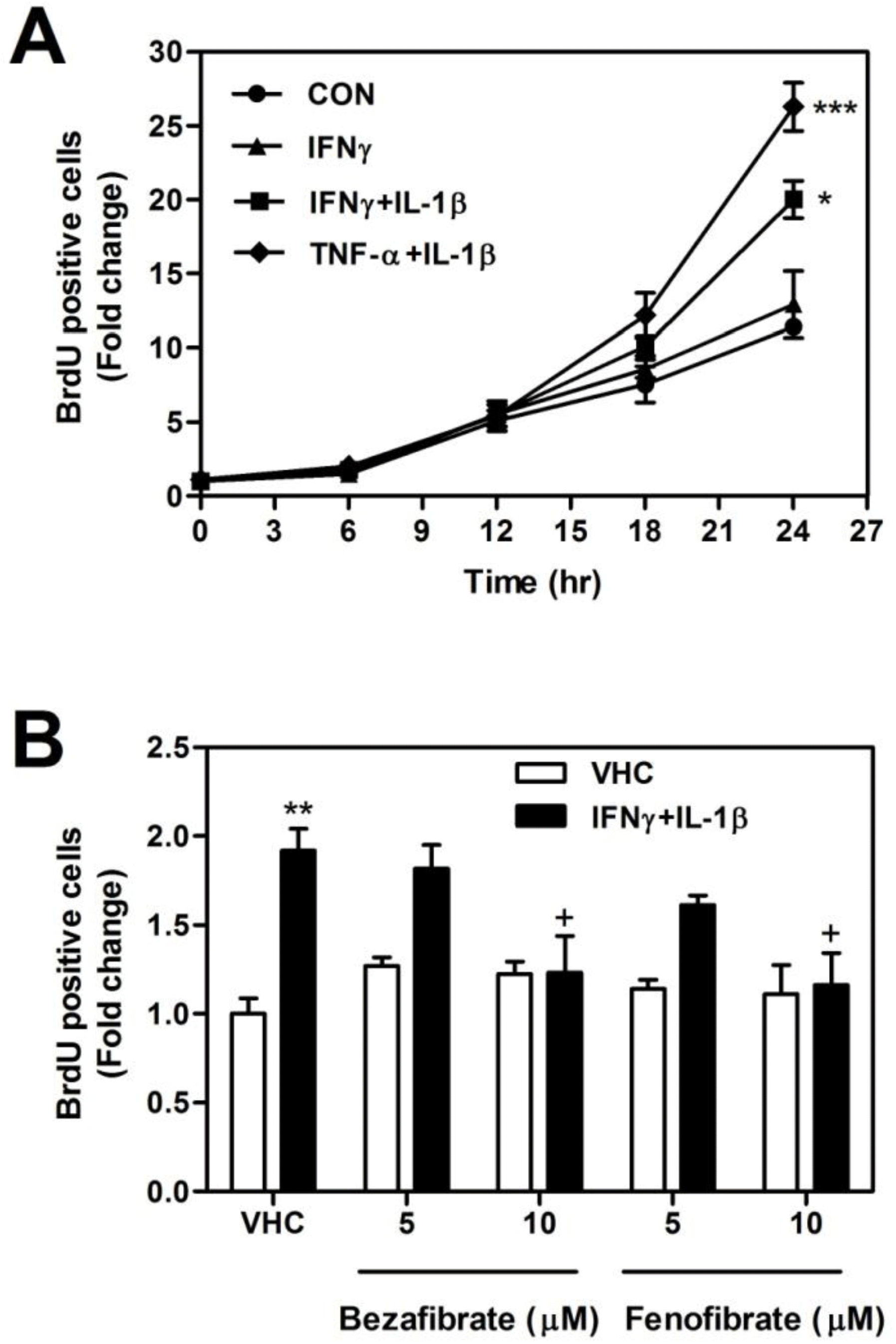 | Fig. 1.Effect of bezafibrate and fenofibrate on the cell proliferation in IFNγ and IL-1β-treated astrocytes. Cells were treated with TNF-α (10 ng/ml), IFN-γ (10 ng/ml), and IL-1β (10 ng/ml). (A) The effect of cytokines on astrocytes proliferation, assayed by BrdU incorporation, was examined at each time point (0, 6, 12, 18, and 24 hr) following alone or co-stimulation with cytokines. (B) The effect of fibrates on cell proliferation was assayed. Cells were preteated with bezafibrate and fenofibrate (0, 5, and 10 μM) for 0.5 hr before stimulation with IFN-γ and IL-1β. Data are represented as mean±S.D. from three independent experiments (∗∗p<0.01; vehicle vs. IFN-γ and IL-1β-treated group, +p<0.05; IFN-γ and IL-1β vs. IFN-γ and IL-1β plus fibrates-treated group). |
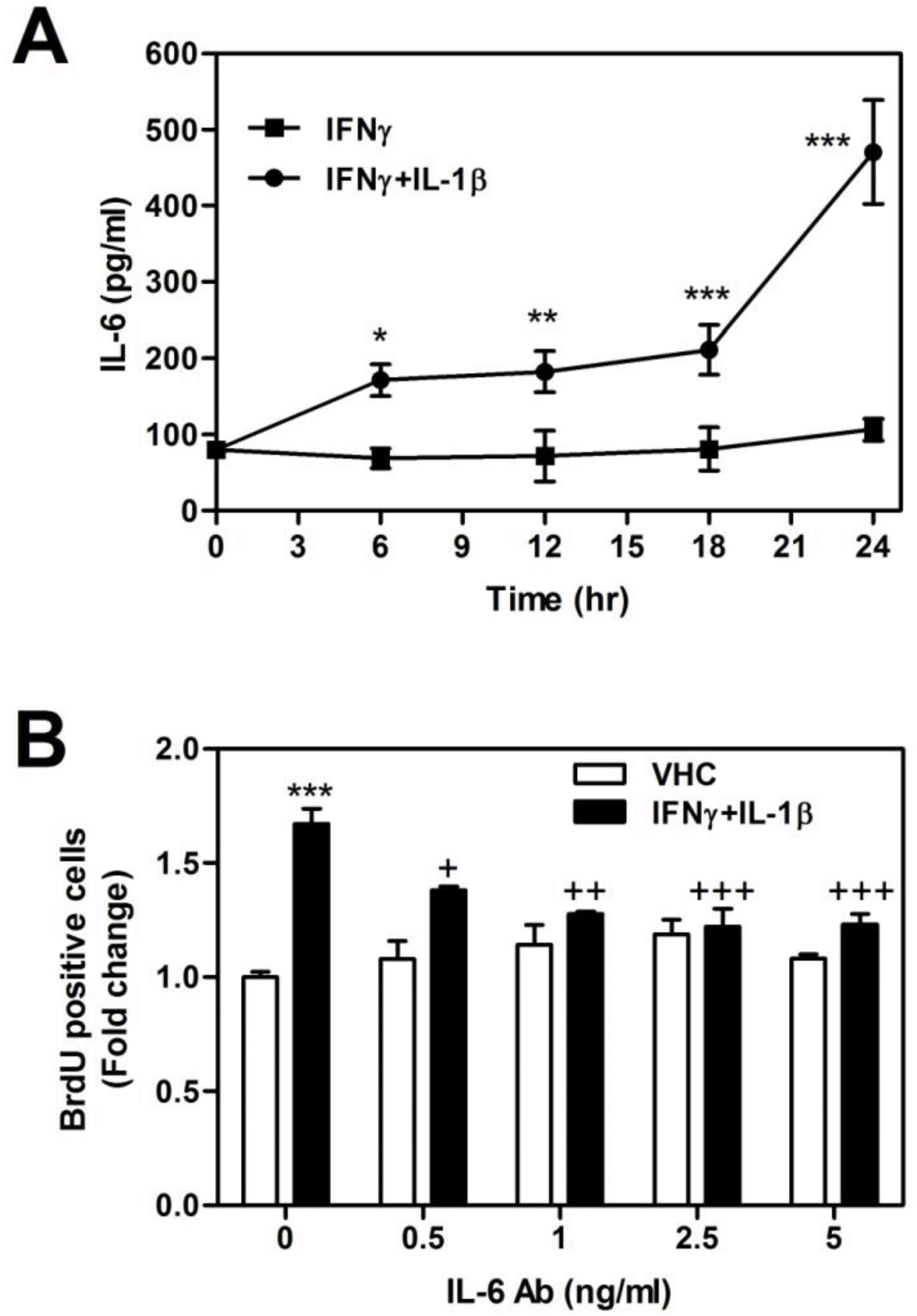 | Fig. 2.Involvement of IL-6 on the cell proliferation in IFNγ and IL-1β-treated astrocytes. Cells were treated with IFN-γ (10 ng/ml) and IL-1β (10 ng/ml). (A) Released IL-6 Protein levels were measured using IL-6 ELISA kit from cultured media after stimulation. Values are mean±S.D. (∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001; IFN-γ vs. IFN-γ and IL-1β). (B) The effect of antagonizing of IL-6 using antibody on cell proliferation was assayed. Cells were preteated with IL-6 antibody (sodium azaide free) (0, 0.5, 1, 2.5, and 5 ng/ml) for 0.5 hr before stimulation with IFN-γ and IL-1β. Data are represented as mean±S.D. from three independent experiments (∗∗∗p<0.001; vehicle vs. IFN-γ and IL-1β-treated group, +p<0.05, ++p<0.01, +++p<0.001; IFN-γ and IL-1β vs. IFN-γ and IL-1β plus IL-6 Ab-treated group). |
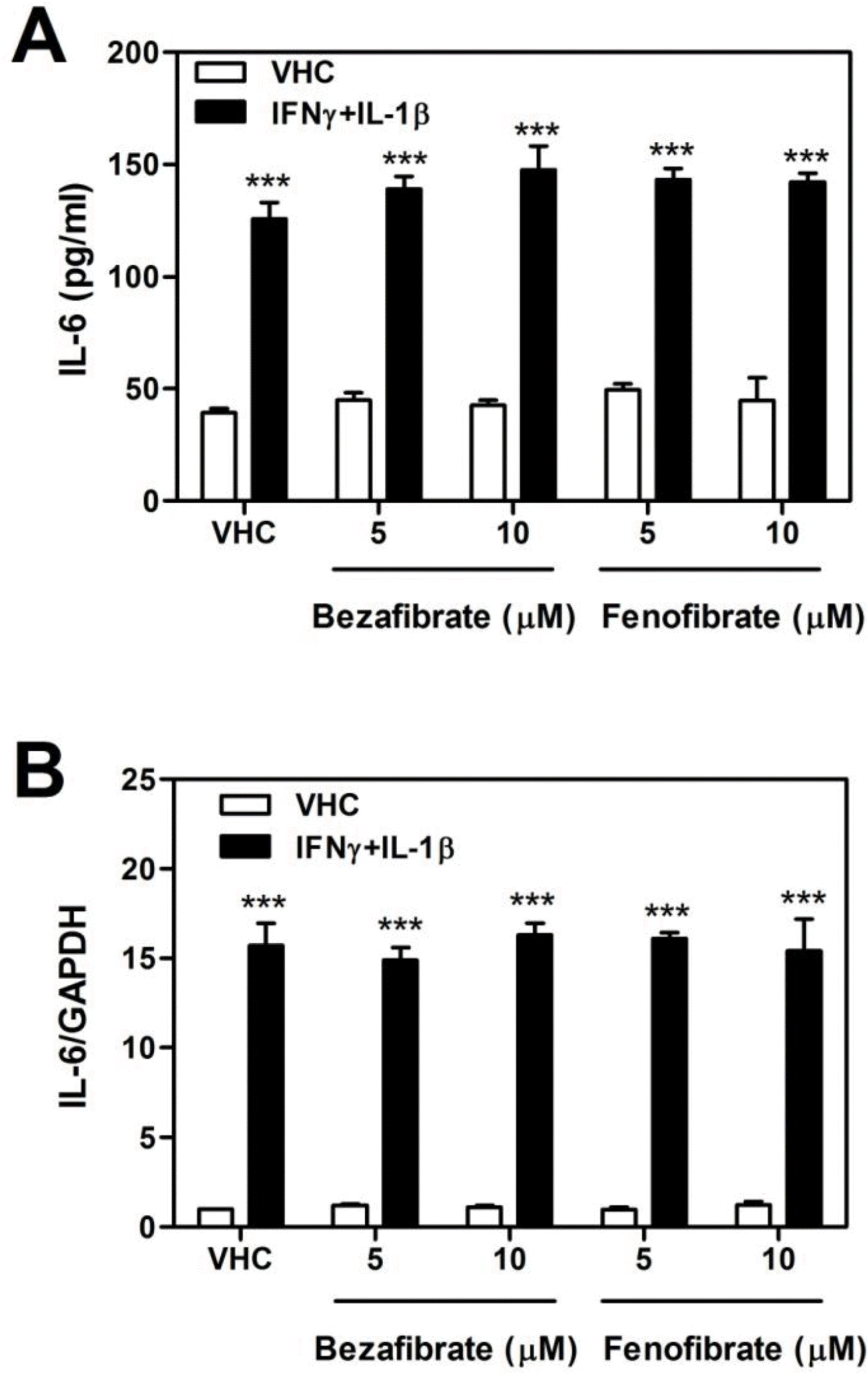 | Fig. 3.Effect of bezafibrate and fenofibrate on the expression of IL-6 in IFNγ and IL-1β-treated astrocytes. Cells were treated with IFN-γ (10 ng/ml) and IL-1β (10 ng/ml). (A) Released IL-6 Protein levels were measured using IL-6 ELISA kit from cultured media after stimulation. (B) Total RNA was isolated from 6 hr in IFN-γ and IL-1β-treated astrocytes. Gene expression of IL-6 was measured by quantitative real-time PCR using Rotor-Gene Q (Qiagen). The expression of IL-6 gene was normalized with GAPDH gene expression. Data were obtained from triplicated IL-6 ELISA and PCR reactions with three different cultures, and values are mean±S.D. (∗∗∗p<0.001; vehicle vs. IFN-γ and IL-1β). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download