Abstract
This study investigated the effects of the methanol extracts of Morinda citrifolia containing numerous anthraquinone and iridoid on phospholipase A2 (PLA2) isozyme. PLA2 activity was measured using various PLA2 substrates, including 10-pyrene phosphatidylcholine, 1-palmitoyl-2-[14C]arachidonyl phosphatidylcholine ([14C]AA-PC), and [3H]arachidonic acid (AA). The methanol extracts suppressed melittin-induced [3H]AA release in a concentration-dependent manner in RAW 264.7 cells, and inhibited cPLA2/sPLA2-induced hydrolysis of [14C]AA-PC in a concentration- and time-dependent manner. A Dixon plot showed that the inhibition by methanol extracts on cPLA2 and sPLA2 appeared to be competitive with inhibition constants (Ki) of 3.7μg/ml and 12.6μg/ml, respectively. These data suggest that methanol extracts of Morinda citrifolia inhibits both Ca2+-dependent PLA2 such as, cPLA2 and sPLA2. Therefore, Morinda citrifolia may possess anti-inflammatory activity secondary to Ca2+-dependent PLA2 inhibition.
Go to : 
References
1. Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994; 269:13057–13060.
2. Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002; 68–69:3–58.
3. Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2s are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998; 273:14411–14423.
4. Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999; 39:175–189.
5. Balsinde J, Dennis EA. Function and inhibition of intracellular calcium-independent phospholipase A2. J Biol Chem. 1997; 272:16069–16072.
6. Potterat O, Hamburger M. Morinda citrifolia (Noni) fruit–phytochemistry, pharmacology, safety. Planta Med. 2007; 73:191–199.
7. Soloman N. The tropical fruit with 101 medicinal uses, NONI juice. Woodland Publishing. 1999.
8. Kamiya K, Tanaka Y, Endang H, Umar M, Satake T. New anthraquinone and iridoid from the fruits of Morinda citrifolia. Chem Pharm Bull (Tokyo). 2005; 53:1597–1599.

9. Balboa MA, Balsinde J, Johnson CA, Dennis EA. Regulation of arachidonic acid mobilization in lipopolysaccharide-activated P388D(1) macrophages by adenosine triphosphate. J Biol Chem. 1999; 274:36764–36768.

10. Radvanyi Fi, Jordan L, Russo-Marie Fi, Bon C. A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal Biochem. 1989; 177:103–109.
11. Wichmann O, Gelb MH, Schultz C. Probing phospholipase A2 with fluorescent phospholipid substrates. Chembiochem. 2007; 8:1555–1569.
12. Martinez J, Moreno JJ. Role of Ca2+-Independent phospholipase A2 on arachidonic acid release Induced by reactive oxygen species. Arch Biochem Biophys. 2001; 392:257–262.
13. Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995; 270:445–450.
14. Fonteh AN, Bass DA, Marshall LA, Seeds M, Samet JM, Chilton FH. Evidence that secretory phospholipase A2 plays a role in arachidonic acid release and eicosanoid biosynthesis by mast cells. J Immunol. 1994; 152:5438–5446.
15. Riendeau D, Guay J, Weech PK, Laliberte F, Yergey J, Li C, Desmarais S, Perrier H, Liu S, Nicoll-Griffith D, Street IP. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J Biol Chem. 1994; 269:15619–15624.
16. Dole VP, Meinertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960; 235:2595–2599.

17. Song HS, Kim HR, Kim MC, Hwang YH, Sim SS. Lutein is a Competitive Inhibitor of Cytosolic Ca2+-dependent Phospholipase A2. J Pharm Pharmacol. 2010; 62:221–227.
18. Lusa S, Myllarniemi M, Volmonen K, Vauhkonen M, Somerharju P. Degradation of pyrene-labelled phospholipids by lysosomal phospholipases in vitro. Dependence of degradation on the length and position of the labelled and unlabelled acyl chains. Biochem J. 1996; 315:947–952.

19. Nielsen OH, Bouchelouche PN, Berild D. Arachidonic acid and calcium metabolism in rnelittin stimulated neutrophils. Mediators Inflamm. 1992; 1:313–317.

20. Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996; 1302:55–60.
21. Bartoli F, Lin HK, Ghomashchi F, Gelb MH, Jain MK, Apitz-Castro R. Tight binding inhibitors of 85-kDa phospholipase A2 but not 14-kDa phospholipase A2 inhibit release of free arachidonate in thrombin-stimulated human platelets. J Biol Chem. 1994; 269:15625–15630.
22. Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993; 32:5935–5940.
23. McKoy ML, Thomas EA, Simon OR. Preliminary investigation of the anti-inflammatory properties of an aqueous extract from Morinda citrifolia (noni). Proc West Pharmacol Soc. 2002; 45:76–78.
Go to : 
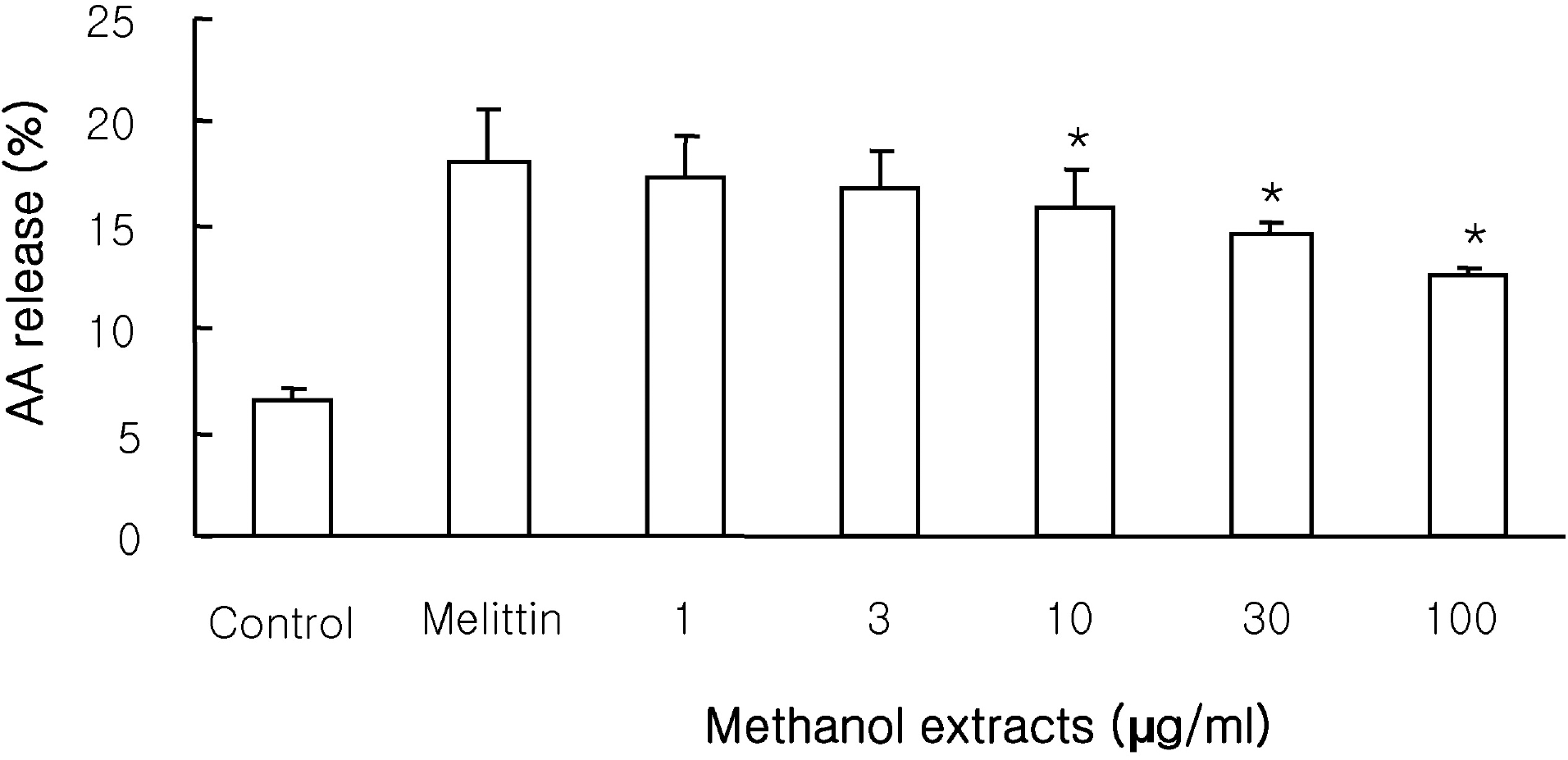 | Fig. 1.Effects of the methanol extracts on [3H]arachidonic acid (AA) release in RAW 264.7 cells stimulated by 0.5 μM melittin. Cells were incubated with the methanol extracts at 37°C for 10 min and AA release was induced by 0.5 μM melittin. Results are mean±S.D. values from 4 separate experiments. ∗p<0.05 vs melittin. |
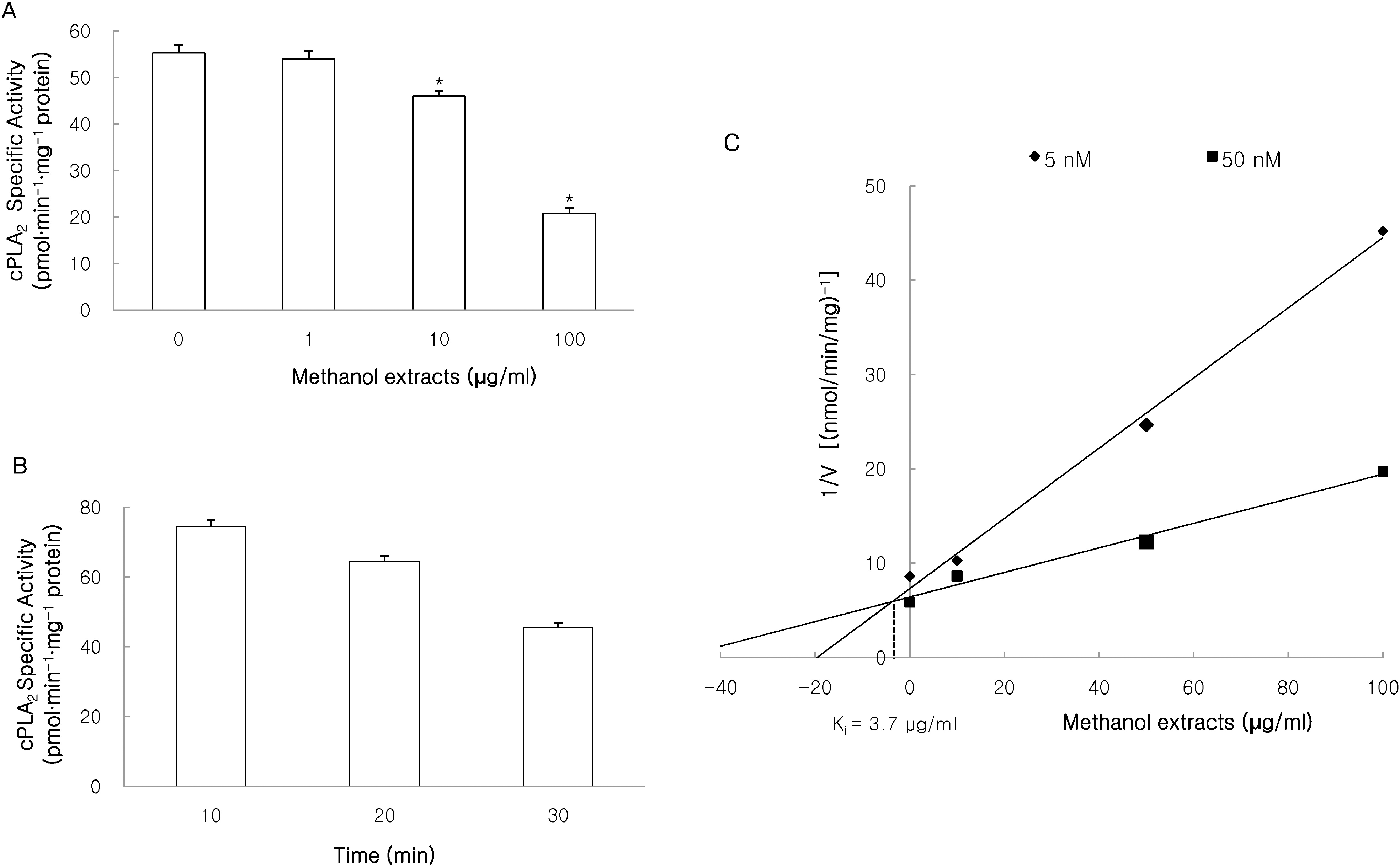 | Fig. 2.Effects of methanol extracts on cPLA2 activity. cPLA2 activity was measured using 1-palmitoyl-2-[14C]arachidonyl phosphatidylcholine ([14C]AA-PC) as a substrate by previously methods. The methanol extracts inhibited cPLA2-induced hydrolysis of [14C]AA-PC in a concentration (A)- and time (B)-dependent manner. A Dixon plot showed that cPLA2 inhibition by methanol extracts appeared to be competitive with an inhibition constant (Ki) of 3.7 μg/ml (C). Results are mean±S.D. values from 4 separate experiments. ∗p<0.05 vs control. |
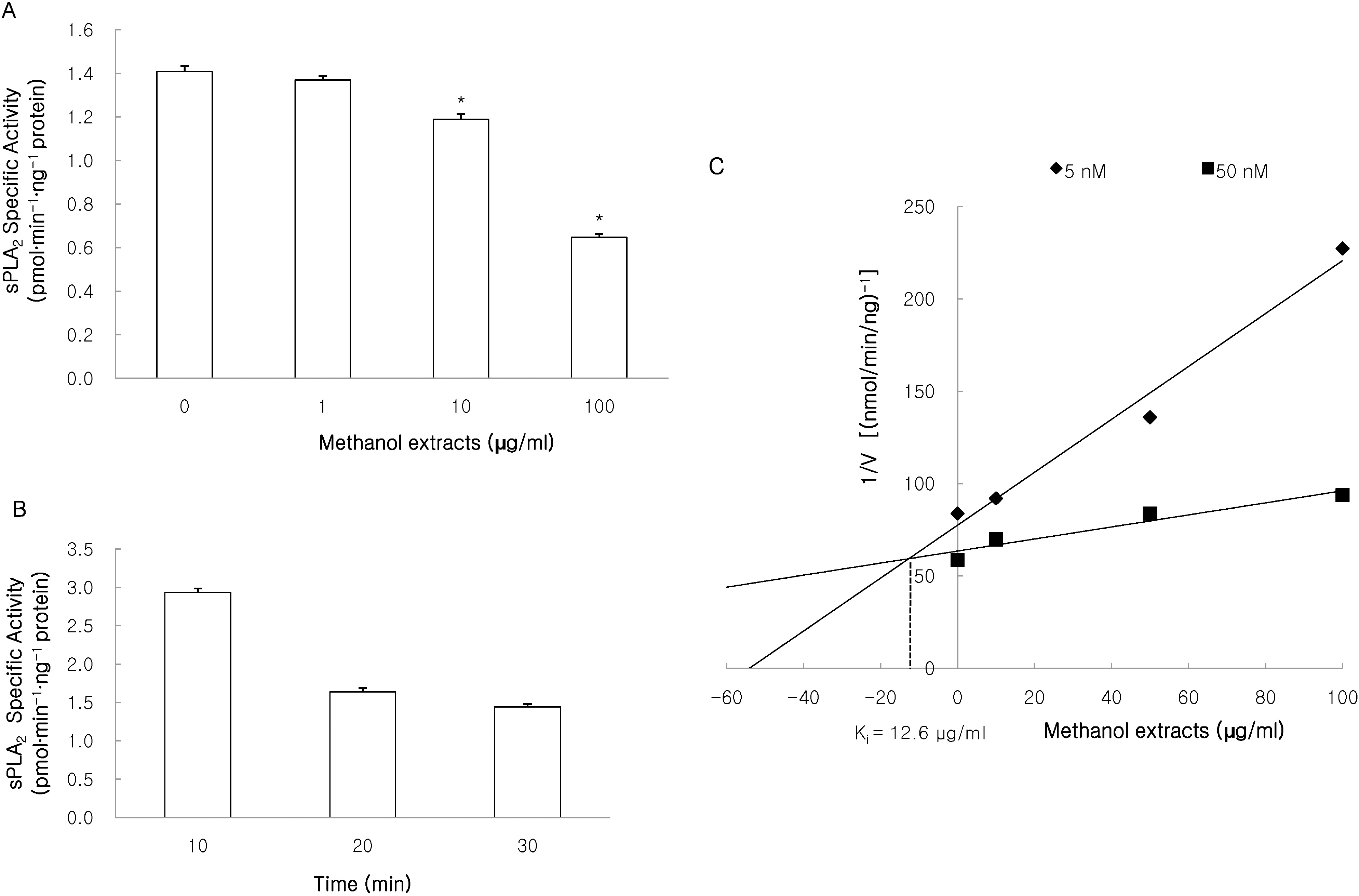 | Fig. 3.Effects of methanol extracts on sPLA2 activity. sPLA2 activity was measured using 1-palmitoyl-2-[14C]arachidonyl phosphatidylcholine ([14C]AA-PC) as a substrate by previously described methods. The methanol extracts inhibited sPLA2-induced hydrolysis of [14C]AA-PC in a concentration (A)- and time (B)-dependent manner. A Dixon plot showed that the inhibition of sPLA2 by methanol extracts appeared to be competitive with an inhibition constant (Ki) of 12.6 μg/ml (C). Results are mean±S.D. values from 4 separate experiments. ∗p<0.05 vs control. |
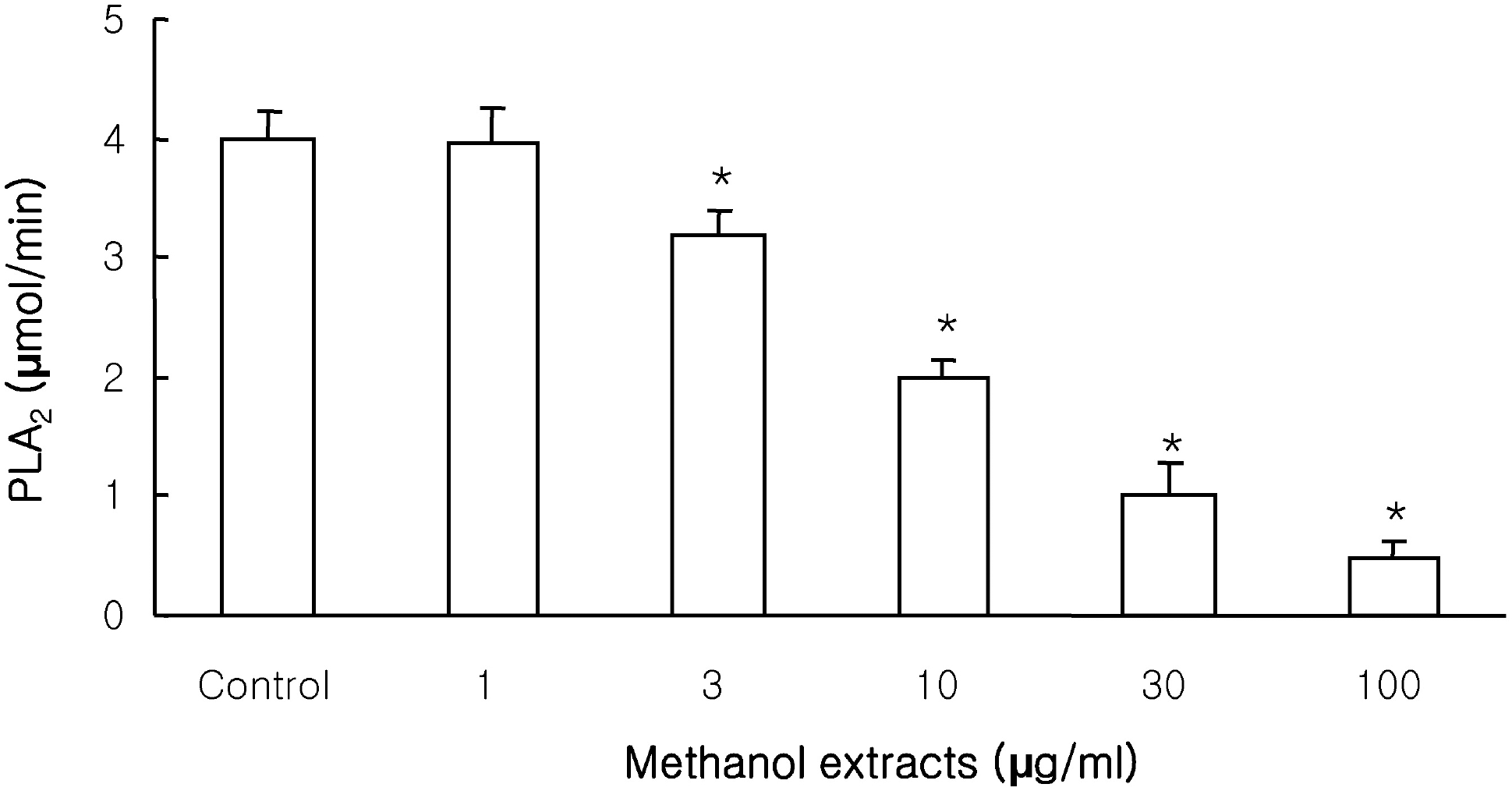 | Fig. 4.Effects of methanol extracts on bee venom sPLA2 activity with 10-pyrene phosphatidylcholine (10-Pyrene PC). 10-Pyrene PC hydrolyzed by purified sPLA2 was inhibited by the methanol extracts in a concentration-dependent manner. Results are mean± S.D. values from 4 separate experiments. ∗p<0.05 vs control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download