Abstract
The sensory system is developed and optimized by experiences given in the early phase of life in association with other regions of the nervous system. To date, many studies have revealed that deprivation of specific sensory experiences can modify the structure and function of the central nervous system; however, the effects of sensory overload remains unclear. Here we studied the effect of overloading the taste sense in the early period of life on the synaptic plasticity of rat hippocampus and somatosensory cortex. We prepared male and female Sprague Dawley rats with ad libitum access to a 0.1% saccharin solution for 2 hrs per day for three weeks after weaning on postnatal day 22. Saccharin consumption was slightly increased in males compared with females; however, saccharin intake did not affect chow intake or weight gain either in male or in female rats. We examined the effect of saccharin-intake on long term potentiation (LTP) formation in hippocampal Schaffer collateral pathway and somatosensory cortex layer IV – II/III pathways in the 6-week old saccharin-fed rats. There was no significant difference in LTP formation in the hippocampus between the control group and saccharin-treated group in both male and female rats. Also in the somatosensory cortex, we did not see a significant difference in LTP among the groups. Therefore, we conclude that saccharin-intake during 3∼6 weeks may not affect the development of physiological function of the cortical and hippocampal synapses in rats.
References
1. Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009; 71:307–332.

3. Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970; 206:419–436.

4. Draeger UC. Observations on monocular deprivation in mice. J Neurophysiol. 1978; 41:28–42.
5. Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000; 10:138–145.

6. Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004; 44:917–923.

7. Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005; 69:215–237.

8. Sotnikov OS. Primary sensory neurons in the central nervous system. Neurosci Behav Physiol. 2006; 36:541–548.

9. Johnson BA, Woo CC, Zeng Y, Xu Z, Hingco EE, Ong J, Leon M. Prolonged stimulus exposure reveals prolonged neurobehavioral response patterns. J Comp Neurol. 2010; 518:1617–1629.

10. Mitchell DE, Sengpiel F. Neural mechanisms of recovery following early visual deprivation. Philos Trans R Soc Lond B Biol Sci. 2009; 364:383–398.

11. Knudsen EI. Experience alters the spatial tuning of auditory units in the optic tectum during a sensitive period in the barn owl. J Neurosci. 1985; 5:3094–3109.

12. Nakahara H, Zhang LI, Merzenich MM. Specialization of primary auditory cortex processing by sound exposure in the “critical period”. Proc Natl Acad Sci U S A. 2004; 101:7170–7174.

13. Smith JC. Microstructure of the rat's intake of food, sucrose and saccharin in 24-hour tests. Neurosci Biobehav Rev. 2000; 24:199–212.

14. Choi SY, Chang J, Jiang B, Seol GH, Min SS, Han JS, Shin HS, Gallagher M, Kirkwood A. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci. 2005; 25:11433–11443.

16. Porciatti V, Bonanni P, Fiorentini A, Guerrini R. Lack of cortical contrast gain control in human photosensitive epilepsy. Nat Neurosci. 2000; 3:259–263.

17. Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003; 300:498–502.

18. Noreña AJ, Gourèvitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006; 9:932–939.

19. Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009; 139:234–244.

20. Collier G, Novell K. Saccharin as a sugar surogate. J Comp Physiol Psychol. 1967; 64:404–408.
21. Smith JC, Sclafani A. Saccharin as a sugar surogate revisited. Appetite. 2002; 38:155–160.
22. Yamamoto T. Cnetral mechanisms of roles of taste in reward and eating. Acta Physiol Hung. 2008; 95:165–186.
23. Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007; 148:1823–1883.

24. Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008; 95:108–113.

25. Zhang TY, Cho HJ, Lee S, Lee JH, Choi SH, Ryu V, Yoo SB, Lee JY, Kim DG, Jahng JW. Impairments in water maze learning of aged rats that received dextromethorphan repeatedly during adolescent period. Psychopharmacology. 2007; 191:171–179.

26. Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region-and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse. 2001; 40:1–10.
27. Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: Gender-dependent effects. Brain Res. 2006; 1097:123–132.

28. Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Devl Neurosci. 2008; 26:259–268.

29. Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006; 9:1001–1003.

Fig. 1.
Daily saccharin intake and body weight of male and female rats. (A) Amount of saccharin solution consumed during daily drinking sessions. Male (circle) and female (triangle) Sprague-Dawley pups had ad libitum access to 0.1% saccharin solution for 2 hr daily for 3 weeks following weaning on postnatal day 22. (B) Total saccharin consumption during each drinking session. (C, D) Body weight gain and food intake of male rat by saccharin intake. Food intake and body weight gain were monitored with (filled circle) or without (open circle) the exposure to 0.1% saccharin for 2 hr daily for 3 weeks during the indicated experimental period. (E, F) Body weight gain and food intake of female rat by saccharin intake. Food intake and body weight gain were monitored with (filled triangle) or without (open triangle) the exposure to 0.1% saccharin for 2 hr daily for 3 weeks during the indicated experimental period. All results are presented as mean±SEM from more than five individual rats.
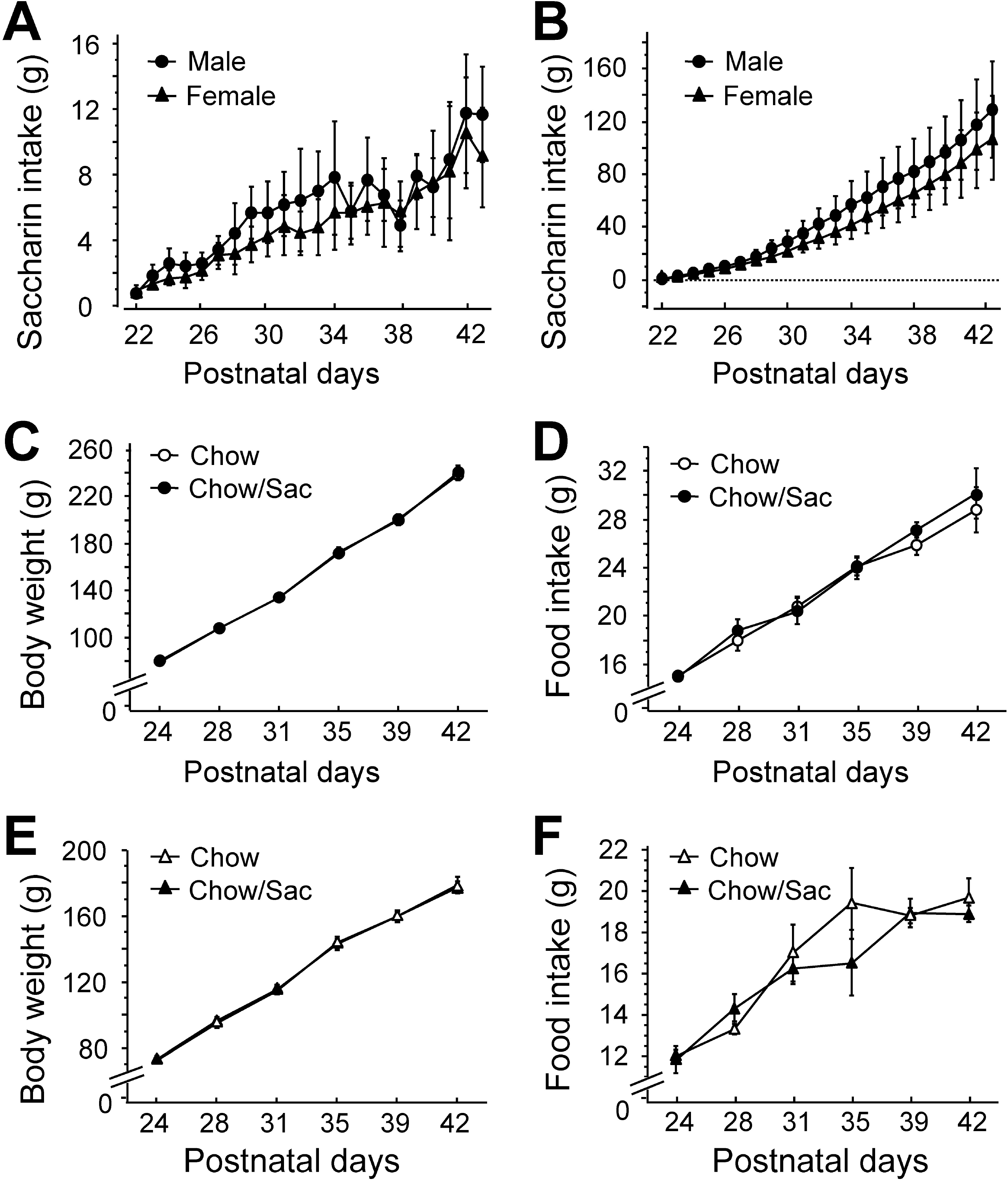
Fig. 2.
Effect of saccharin intake on LTP induction in hippocampal Schaffer collateral pathway in male rat. (A) Hippocampal slices were prepared from 6∼7 weeks old male Sprague-Dawley rat with (filled circle) and without (open circle) ad libitum access to 0.1% saccharin solution for 2 hr daily for 3 weeks, and recorded fEPSP in Schaffer collateral-CA1 synapses. Average changes in the fEPSP slope induced by theta burst stimulation are depicted. (B) Typical field potential traces from experiments performed in saccharine-treated group (right) and chow only group (left) are shown. The superimposed traces are averages of four consecutive responses recorded 1 min before (black traces) and 1 hr after (gray traces) theta burst stimulation. (C) The magnitude of LTP formation of baseline responses is shown depicted. All results are presented as mean±SEM from more than four independent trials. n.s., not significant statistical difference (p>0.05).
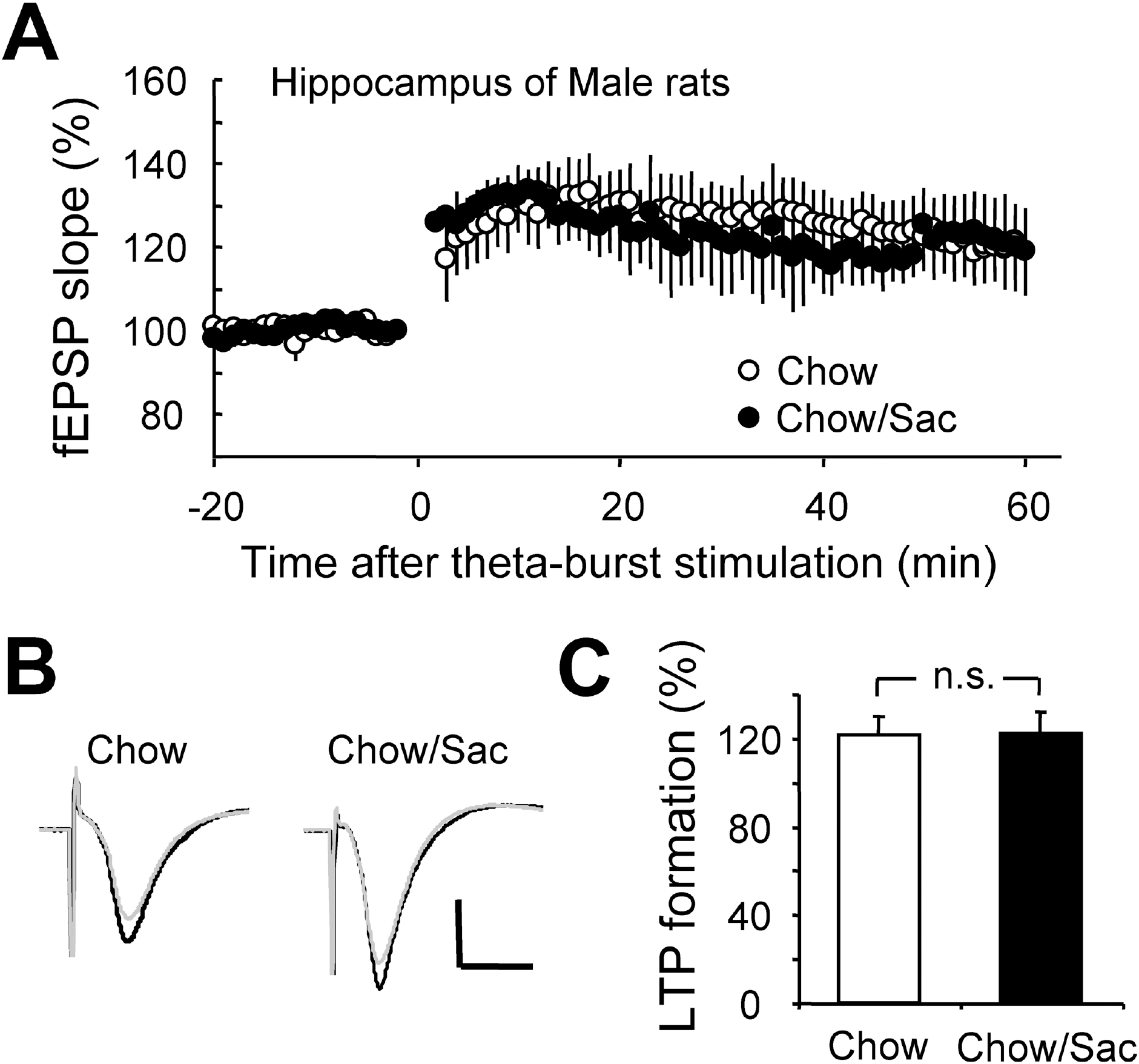
Fig. 3.
Effect of saccharin intake on LTP induction in hippocampal Schaffer collateral pathway in female rat. (A) Hippocampal slices were prepared from 6∼7 weeks old female rat with (filled circle) and without (open circle) 0.1% saccharin intake, and recorded fEPSP in Schaffer collateral-CA1 synapses. Average changes in the fEPSP slope induced by theta burst stimulation are depicted. (B) Typical field potential traces from experiments performed in saccharine-treated group (right) and chow only group (left) are shown. The superimposed traces are averages of four consecutive responses recorded 1 min before (black traces) and 1 hr after (gray traces) theta burst stimulation. (C) The magnitude of LTP formation of baseline responses is shown depicted. All results are presented as mean±SEM from more than five independent trials. n.s., not significant statistical difference (p>0.05).
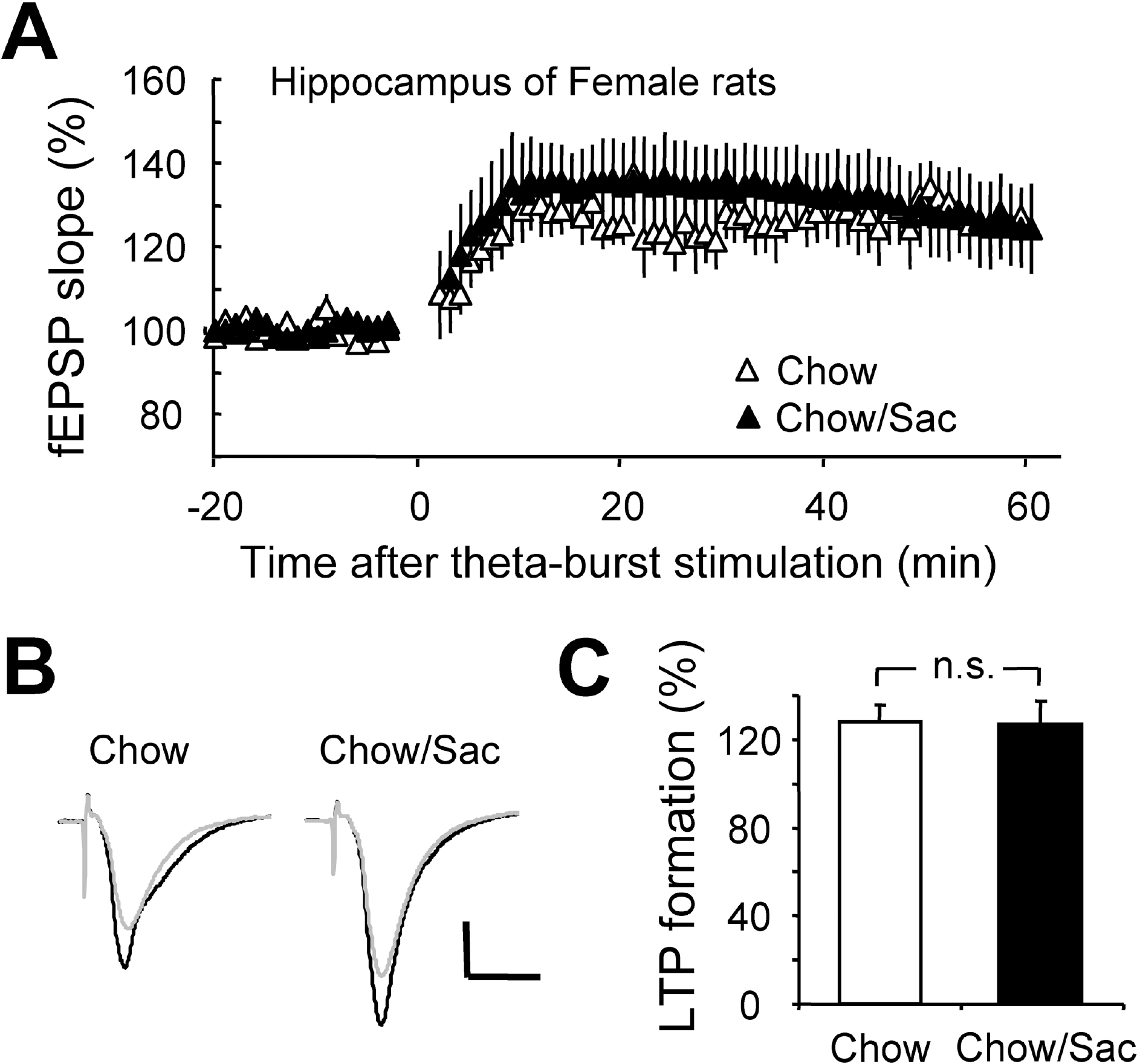
Fig. 4.
Effect of saccharin intake on LTP induction in somatosensory cortex layer IV-II/III pathway in male rat. (A) Somatosensory cortical slices were prepared from 6∼7 weeks old male rat with (filled circle) and without (open circle) 0.1% saccharin intake, and recorded fEPSP in layer IV-II/III synapses. Average changes in the fEPSP amplitude induced by theta burst stimulation are depicted. (B) Typical field potential traces from experiments performed in saccharine-treated group (right) and chow only group (left) are shown. The superimposed traces are averages of four consecutive responses recorded 1 min before (black traces) and 1 hr after (gray traces) theta burst stimulation. (C) The magnitude of LTP formation of baseline responses is shown depicted. All results are presented as mean±SEM from more than three independent trials. n.s., not significant statistical difference (p>0.05).

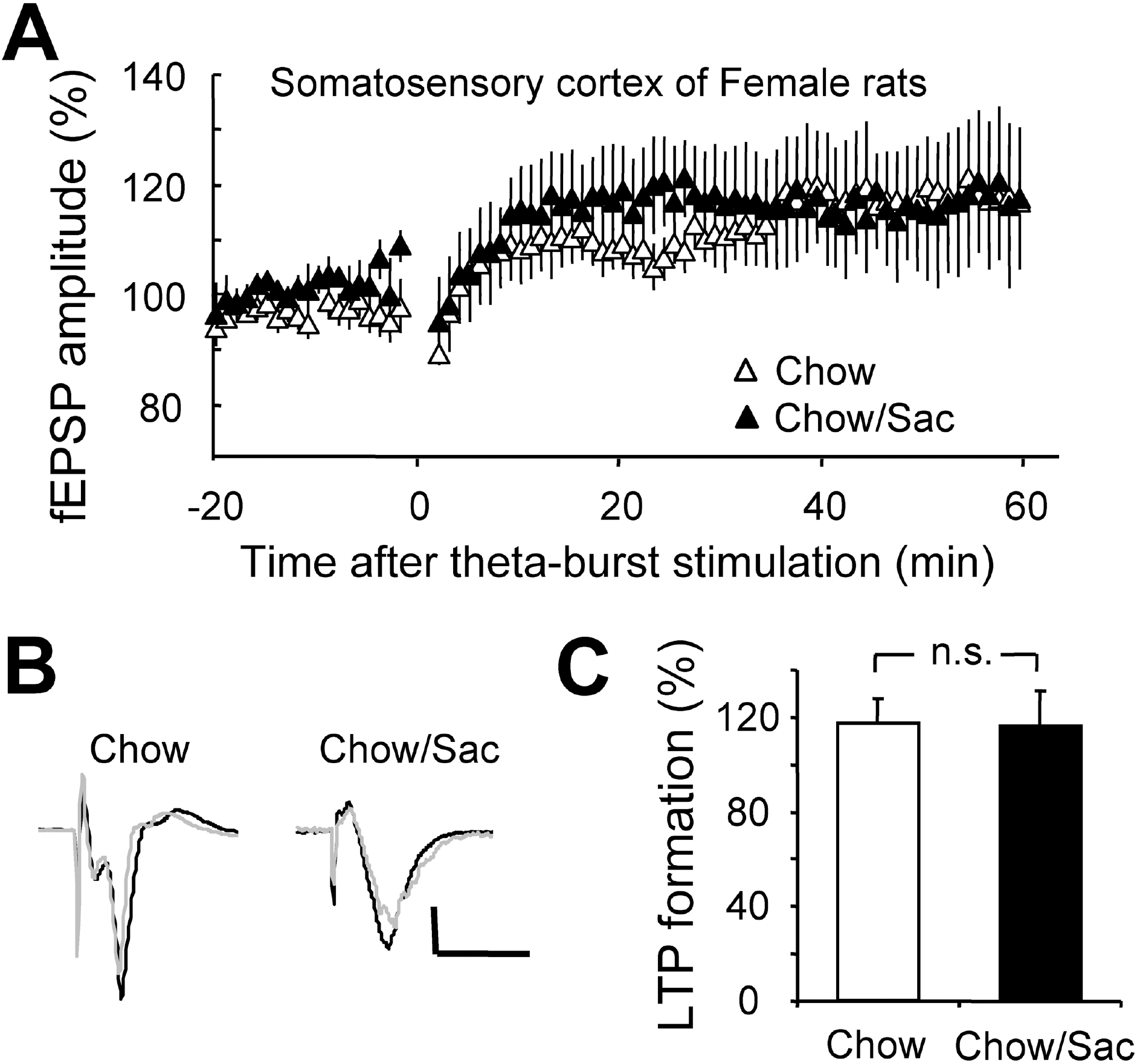
Fig. 5.
Effect of saccharin intake on LTP induction in somatosensory cortex layer IV-II/III pathway in female rat. (A) Somatosensory cortical slices were prepared from 6∼7 weeks old female rat with (filled circle) and without (open circle) 0.1% saccharin intake, and recorded fEPSP in layer IV-II/III synapses. Average changes in the fEPSP amplitude induced by theta burst stimulation are depicted. (B) Typical field potential traces from experiments performed in saccharine-treated group (right) and chow only group (left) are shown. The superimposed traces are averages of four consecutive responses recorded 1 min before (black traces) and 1 hr after (gray traces) theta burst stimulation. (C) The magnitude of LTP formation of baseline responses is shown depicted. All results are presented as mean±SEM from more than three independent trials. n.s., not significant statistical difference (p>0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download