Abstract
Human tumors, including those of the hepatobiliary system, express a number of specific antigens that can be recognized by T cells, and may provide potential targets for cancer immunotherapy. Dendritic cells (DCs) are rare leucocytes that are uniquely potent in their ability to capture, process and present antigens to T cells. The ability to culture sufficient numbers of DCs from human bone marrow or blood progenitors has attracted a great deal of interest in their potential utilization in human tumor vaccination. CD34+ peripheral blood stem cells (PBSCs) were obtained from a patient with a hepatocellular carcinoma. The PBSCs were cultured in the X-VIVO 20 medium supplemented with the Flt-3 Ligand (FL), GM-CSF, IL-4 and TNF-α for 12 days. The morphology and functions of the cells were examined. The generated cells had the typical morphology of DCs. When the DCs were reinjected into the same patient, an augmentation of the cytotoxic T lymphocyte (CTL) activity was observed. Concomitantly, an increase in the natural killer (NK) cell activity was also detected in the patient. These results suggest that DCs-based cancer immunotherapy may become an important treatment option for cancer patients in the future.
References
1. Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991; 9:271–296.

2. Lee DH, Park JS, Eo WK, Kim WM, Kang K. Differentiation induction of dendritic cell phenotypes from human leukemic cell lines. Korean J Physiol Pharmacol. 2001; 5:79–86.
3. McKenna HJ, de Vries P, Brasel K, Lyman SD, Williams DE. Effect of flt3 ligand on the ex vivo expansion of human CD34+ hematopoietic progenitor cells. Blood. 1995; 86:3413–3420.
4. Young CJ, Varma A, DiGiusto D, Backer MP. Retention of quiescent hematopoietic cells with high proliferative potential during ex vivo stem cell culture. Blood. 1996; 87:545–556.

5. Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, Fagioli F, Berger M, Aglietta M. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997; 89:2644–2653.

6. Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckman MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994; 83:2795–2801.

8. Ockert D, Schmitz M, Hampl M, Rieber EP. Advances in cancer immunotherapy. Immunol Today. 1999; 20:63–65.

9. Nestle FO, Burg G, Dummer R. New perspectives on immunobiology and immunotherapy of melanoma. Immunol Today. 1999; 20:5–7.

10. Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997; 18:267–268.

11. Steinman RM. Dendritic cells and immune-based therapies. Exp Hematol. 1996; 24:859–862.
12. Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997; 186:1183–1187.

13. Lotze MT. Getting to the source: dendritic cells as therapeutic reagents for the treatment of patients with cancer. Ann Surg. 1997; 226:1–5.

15. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392:245–252.

16. Bottomly K. Immunology-T cells and dendritic cells get intimate. Science. 1999; 283:1124–1125.
Fig. 1.
Phase contrast micrograph of freshly isolated peripheral blood stem cells showing a homogenous population of equally sized and round cells. Original magnification ×200 (A). After 3 days of culture with the medium containing GM-CSF, IL-4, TNF-α and FL. Spindle-shape cells are shown in the center. Original magnification ×200 (B). After 3 days of culture with the medium. Original magnification ×100 (C). After 6 days of culture with medium containing GM-CSF, IL-4, TNF-α and FL, cells with large-cell bodies and long dendritic projections were visible. Original magnification ×400 (D). After 6 days of culture with medium. Original magnification ×400 (E).
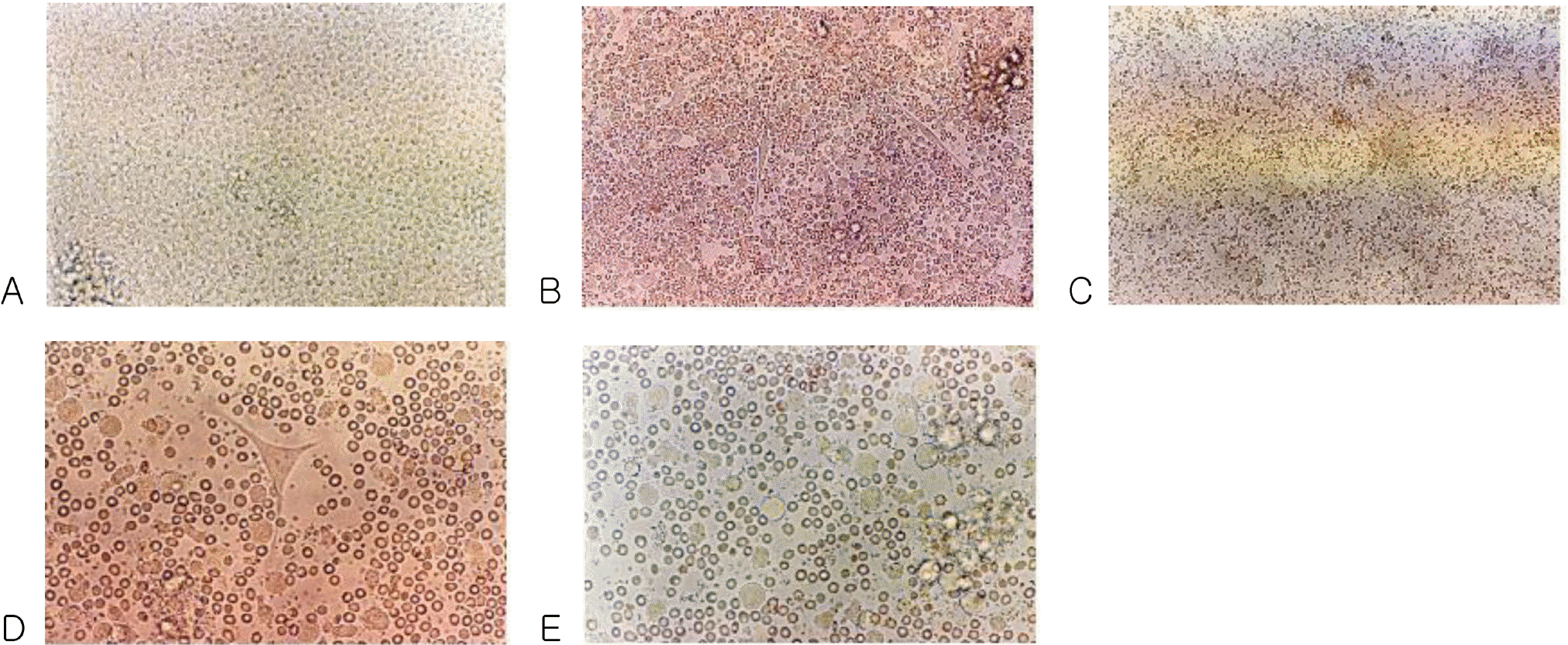
Fig. 2.
Cells with long cytoplasmic projections were visible at 12 days of culture in a medium containing GM-CSF, IL-4, TNF-α and FL. Original magnification ×400.
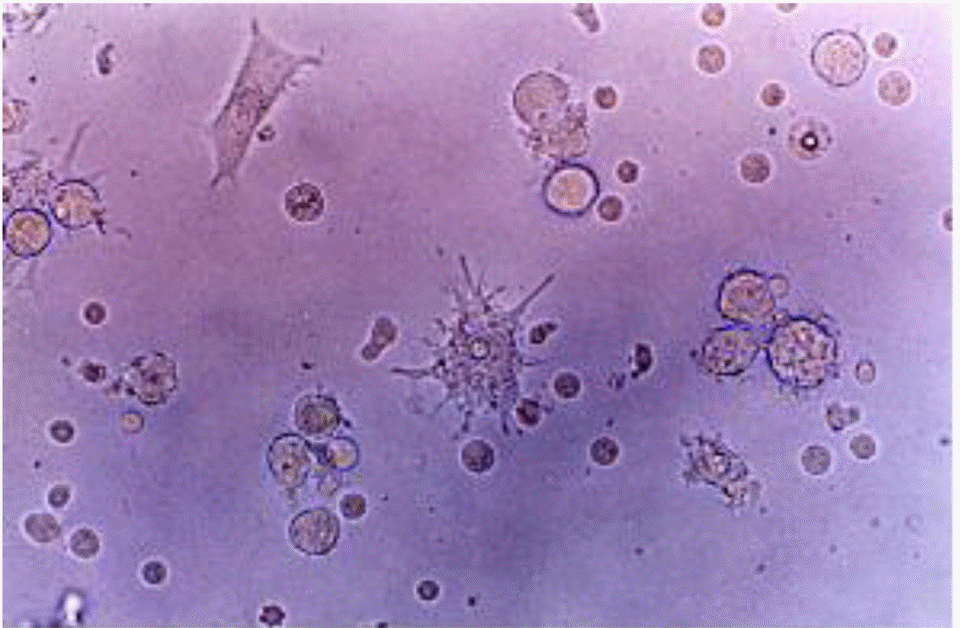
Fig. 3.
Fluorescence micrograph of cultured DCs showing intense staining with the monoclonal antibodies for the specific cell surface immunophenotype. DCs showed positive FITC staining. (A) CD1a, (B) CD83 and (C) CD86.
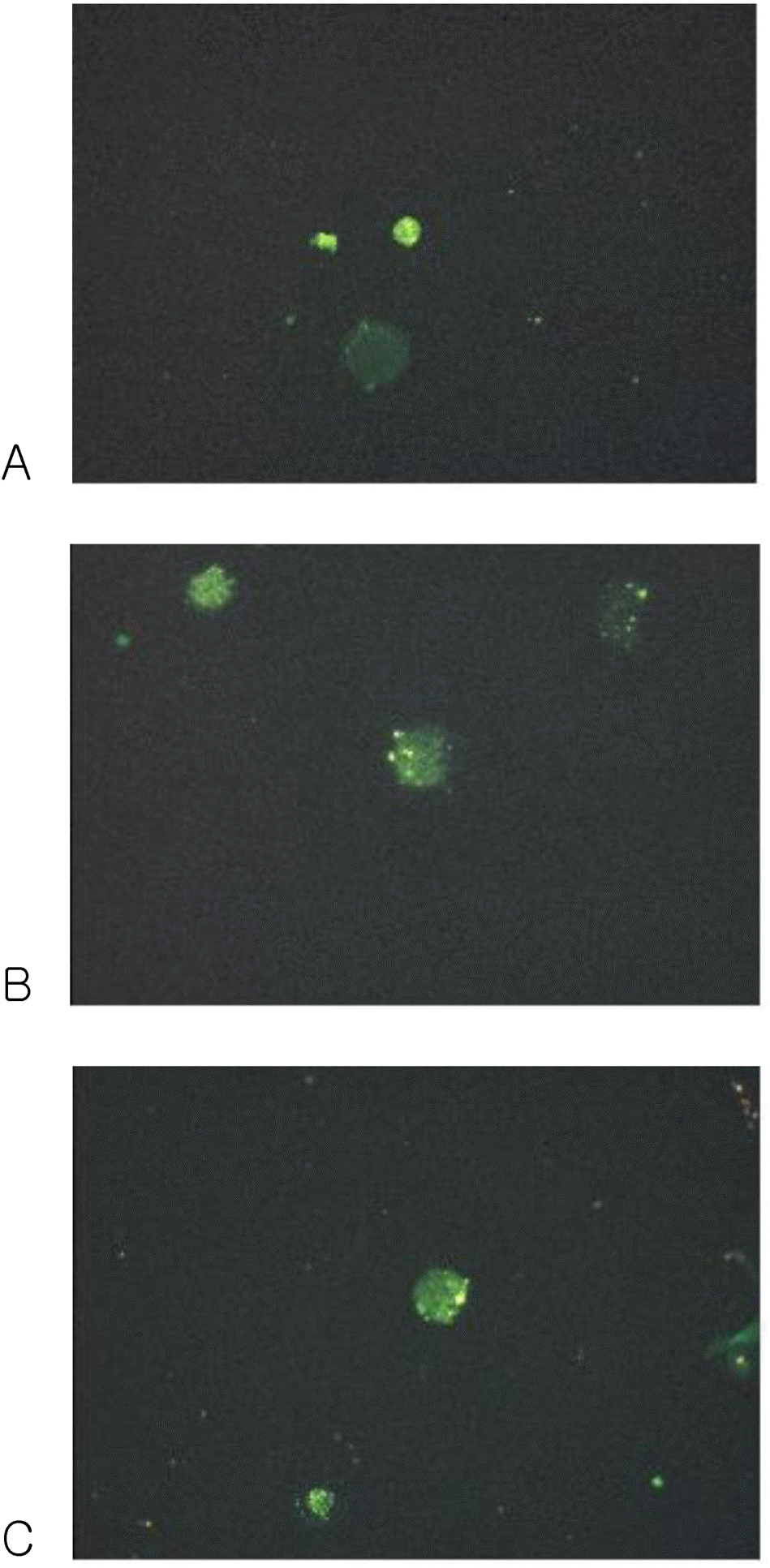
Table 1.
Relative fractions of T and NK cells, and NK cells activity in the peripheral blood of a patient with a hepatocellular carcinoma before and after DCs therapy
| Before DCs therapy | After DCs therapy | |
|---|---|---|
| CD3 (%) | 43.5 | 49.0 |
| CD4 (%) | 28.2 | 27.7 |
| CD8 (%) | 16.2 | 22.0 |
| CD8:CD4 | 0.57: 1 | 0.79: 1 |
| CD56 (%) | 14.8 | 12.4 |
| Activity of NK cells (%) | 15.7 | 28.9 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download