Abstract
Exposure to early stressful adverse life events such as maternal and social separation plays an essential role in the development of the nervous system. Adolescent Sprague-Dawley rats that were separated on postnatal day 14 from their dam and litters (maternal social separation, MSS) showed hyperactivity and anxiolytic behavior in the open field test, elevated plus-maze test, and forced-swim test. Biologically, the number of astrocytes was significantly increased in the prefrontal cortex of MSS adolescent rats. The hyperactive and anxiolytic phenotype and biological alteration produced by this MSS protocol may provide a useful animal model for investigating the neurobiology of psychiatric disorders of childhood-onset diseases, such as attention deficient hyperactive disorder.
REFERENCES
Aisa B., Tordera R., Lasheras B., Del Rio J., Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 154:1218–1226. 2008.

Amen DG., Carmichael BD. High-resolution brain SPECT imaging in ADHD. Ann Clin Psychiatry. 9:81–86. 1997.

Arnold JL., Siviy SM. Effects of neonatal handling and maternal separation on rough-and-tumble play in the rat. Dev Psychobiol. 41:205–215. 2002.

Alward EH., Reiss AL., Reader MJ., Singer HS., Brown JE., Denekla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol. 11:112–115. 1996.

Bremner D., Vermetten E. Stress and development: Behavioral and biological consequences. Dev Psychopath. 13:473–489. 2001.

Choe GJ., Kim SA., Lee HJ., Chung JH., Kim JW. Effects of maternal separation and fluoxetine treatment on the expressions of nitric oxide synthase in hypothalamus of rat brain. Korean J Psychopharmacol. 13:262–268. 2002.
Cirulli F., Berry A., Alleva E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev. 27:73–82. 2003.

Colorado RA., Shumake J., Conejo NM., Gonzalez-Pardo H., Gonzalez- Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behaiv Processes. 71:51–58. 2006.

Daniels WM., Pietersen CY., Carstens ME., Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 19:3–14. 2004.

Elenbroek BA., Riva MA. Early maternal deprivation as an animal model for schizophrenia. Clin Neurosci Res. 6:297–302. 2003.
Fabricius K., Wörtwein G., Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 212:403–416. 2008.

Filipeck PA., Semrud-Clikeman M., Steingard RJ., Renshaw PF., Kennedy DN., Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 48:589–601. 1997.

Fransis DD., Diorio J., Plotsky PM., Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 22:7840–7843. 2002.
Giachino C., Canalia N., Capone F., Fasolo A., Alleva E., Riva MA., Cirulli F., Peretto P. Maternal deprivation and early handling affect density of calcium binding protein-coating neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 16:568–578. 2007.
Holsboer F. Animal models of mood disorders. Charney DS, Nestler EJ, Bunney BS, editors. ed,. Neurobiology of Mental Illness. 1st ed.Oxford University Press;New York: p. p. 317–332. 1999.
Karten YJ., Olariu A., Cameron HA. Stress in early life inhibits neurogenesis in adulthood. Trends Neurosci. 28:171–172. 2005.

Kwak HR., Lee JW., Kwon K., Park J., Chun W., Kim SS., Lee HJ. Neuronal architecture of the hippocampal formation and cerebral cortex in maternal social separation rats. Clin Psychopharm Neurosci. 6:65–70. 2008.
Lee HJ., Kim JW., Yim SV., Kim MJ., Kim SA., Kim YJ., Kim CJ., Chung JH. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 6:725–728. 2001.

Lee HJ., Son CH., Kwak HR., Lee SH., Han YH., Kim SY., Park JI., Chun W., Kim SS. Microarray analysis of gene expression in rat hippocampus of maternal social separation model. Korean J Biol Psychiatry. 13:110–116. 2006.
Macri S., Mason CJ., Würbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci. 20:1017–1024. 2004.

Madruga C., Xavier LL., Achaval M., Sanvitto GL., Lucion AB. Early handling, but not maternal separation, decreases emotional responses in two paradigms of fear without changes in mesolimbic dopamine. Behav Brain Res. 166:241–246. 2006.

McEwen BS. Early life influences on life-long patterns of behavior and health. MRDD Res Rev. 9:149–154. 2003.

Mirescu C., Peters JD., Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neurosci. 7:841–846. 2004.

Negishi T., Kawasaki K., Sekiguchi S., Ishii Y., Kyuwa S., Kuroda Y., Yoshikawa Y. Attention-deficit and hyperactive neurobehavioural characteristics induced by perinatal hypothyroidism in rats. Behav Brain Res. 159:323–331. 2005.

Paule MG., Rowland AS., Ferguson SA., Chelonis JJ., Tannock R., Swanon JM., Castellanos FX. Attention deficit/hyperactivity disorder: characteristics, interventions, and models. Neurotoxicol Teratol. 22:631–651. 2000.

Porsolt RD., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 229:327–336. 1977.
Pryce CR., Rüedi-Bettschen D., Detting AC., Weston A., Russig H., Ferger B., Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 29:649–674. 2005.

Rüedi- Bettschen D., Pedersen EM., Feldon J., Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 156:297–310. 2005.
Sagvolden T., Russell VA., Aase H., Johansen EB., Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 57:1239–1247. 2005.

Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 301:805–809. 2003.

Todd RD., Botteron KN. Is attention-deficit/hyperactivity disorder an energy deficiency syndrome? Biol Psychiatry. 50:151–158. 2001.

Fig. 1.
Comparison between controls and maternal social separation (MSS) group on the open-field test. Data are expressed as mean±SEM. ∗∗p<0.01, Significant difference from control (Mann-Whitney U-test).
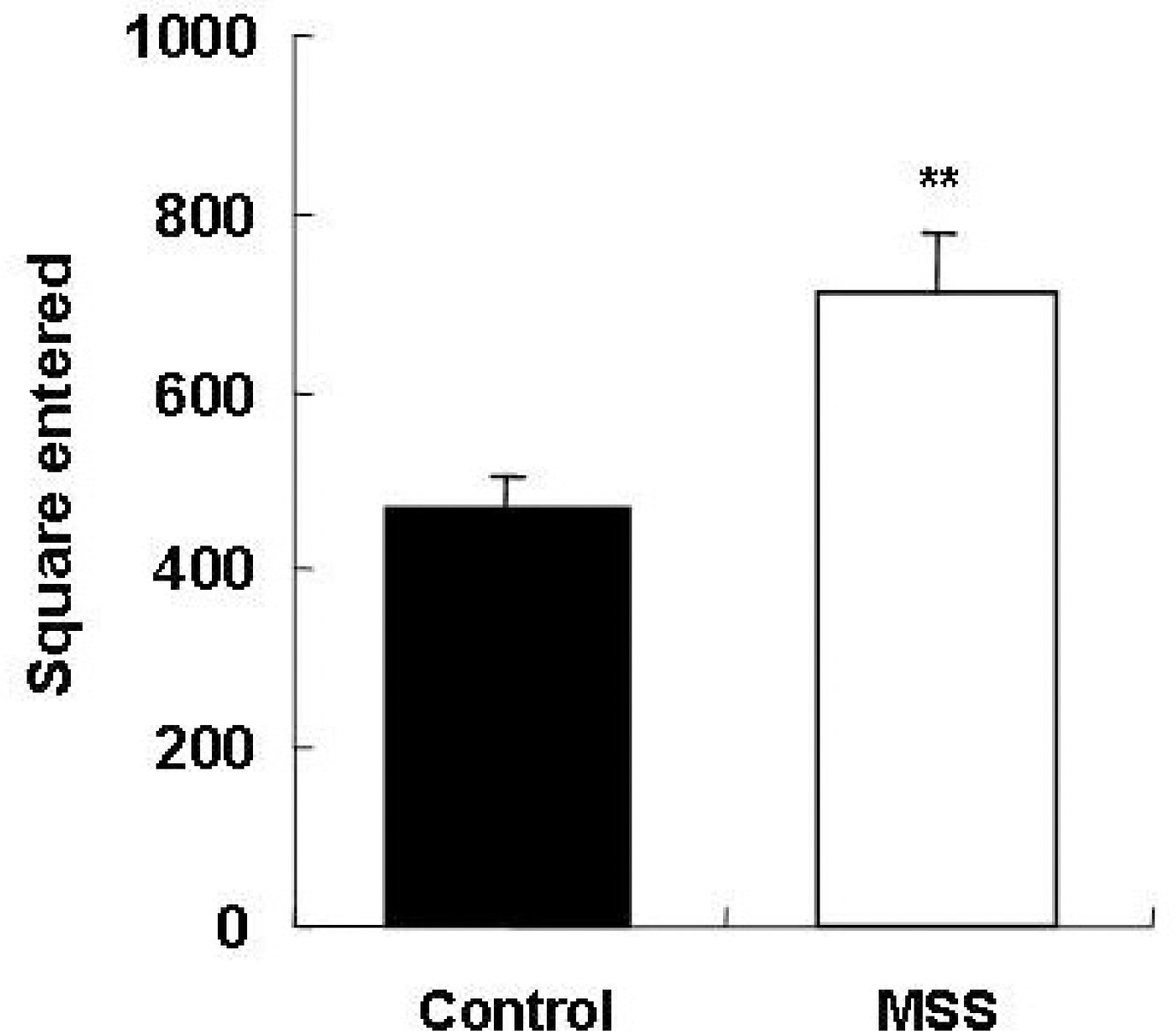
Fig. 2.
Effects of maternal and social separation on the elevated plus-maze test. Anxiety-related behavior as reflected by the percentage of entries (A) and time spent (B) on the open arms of the elevated plus-maze by controls and maternal social separation (MSS) rats on PND 35. Data are expressed as mean±SEM. ∗p<0.05, ∗∗p<0.01, Significant difference from control (Mann- Whitney U-test).
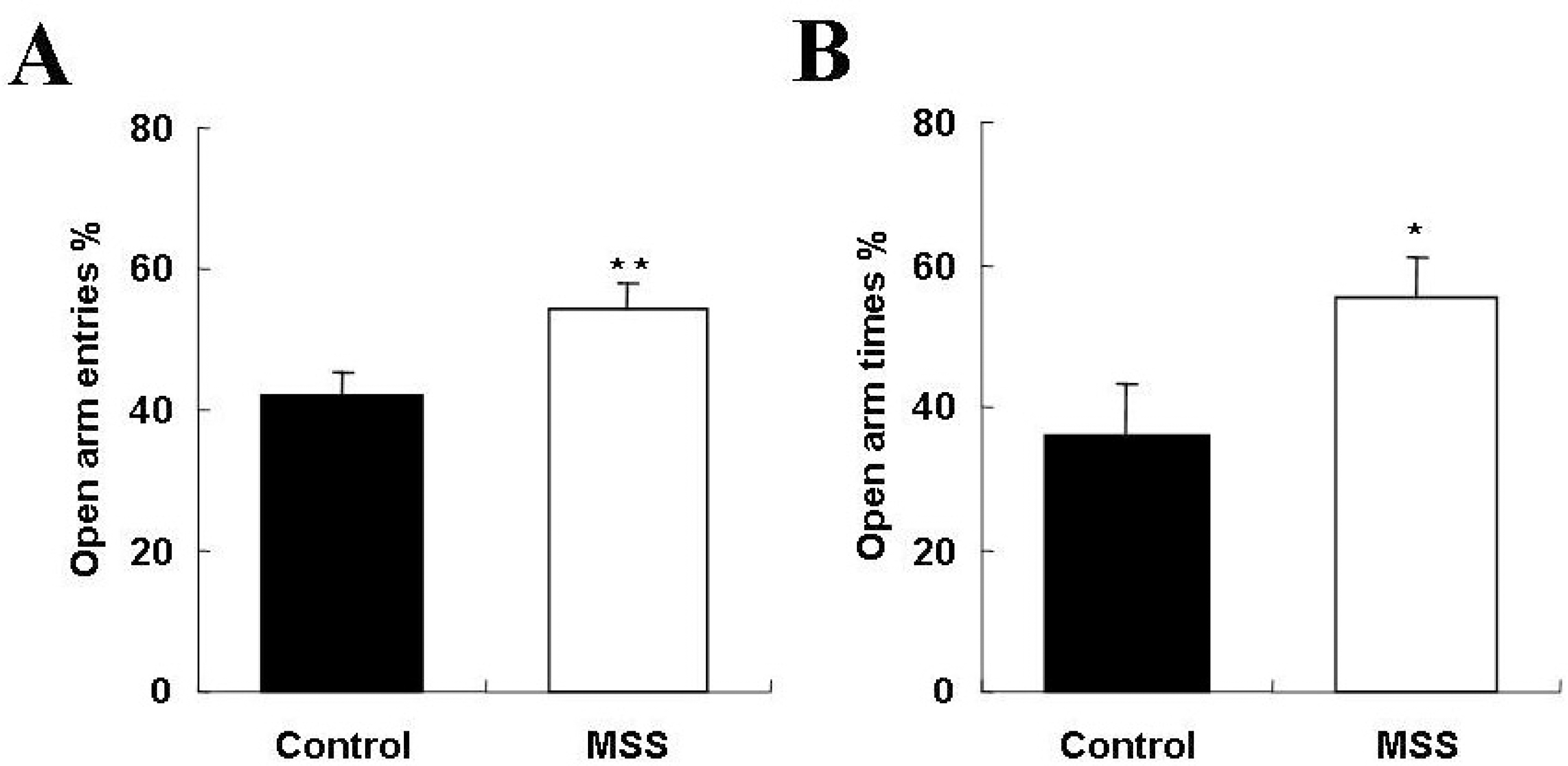
Fig. 3.
Effects of maternal and social separation on the forced swim test. Data are expressed as mean±SEM. ∗∗p<0.01, Significant difference from control (Mann-Whitney U-test).
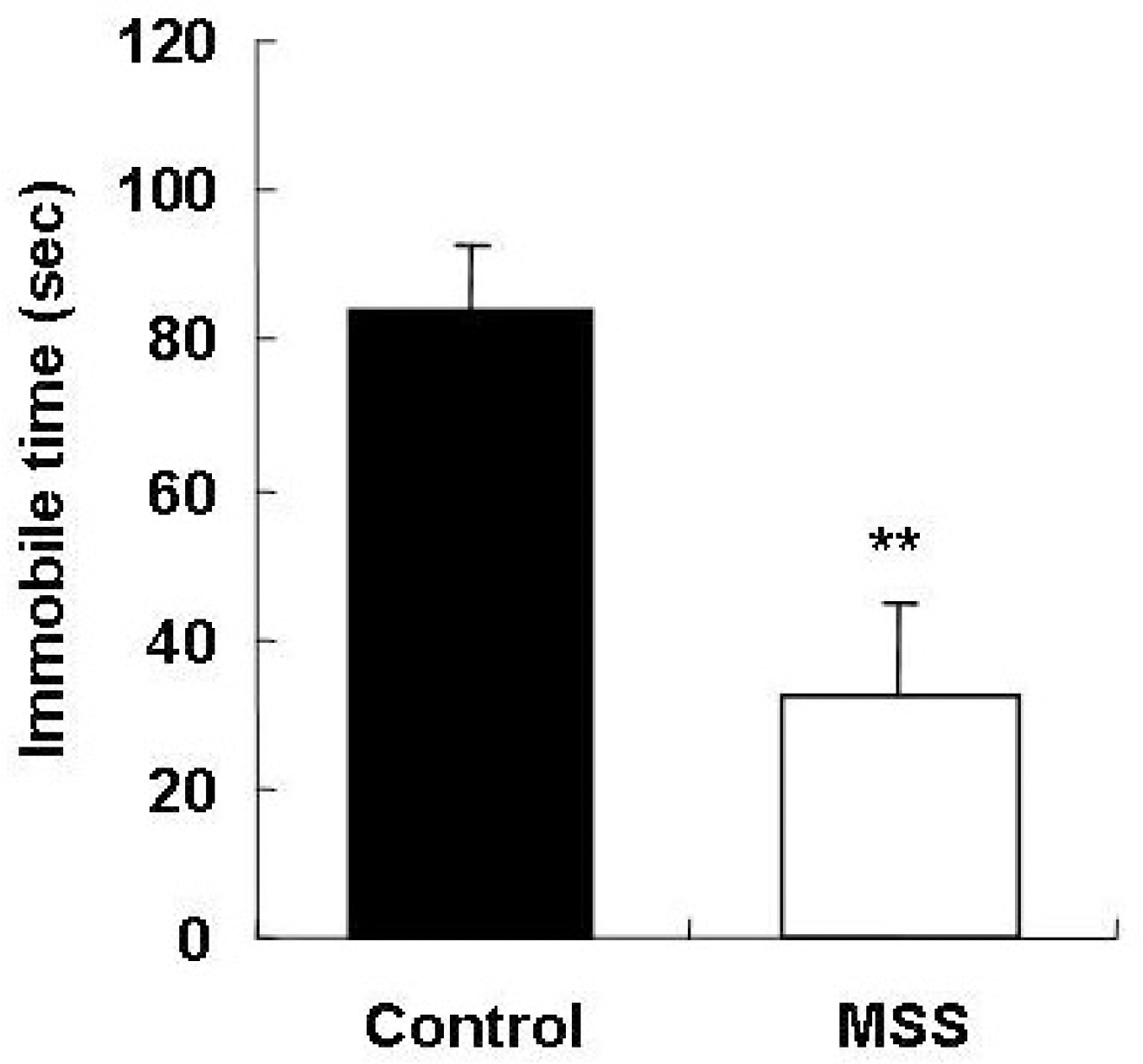
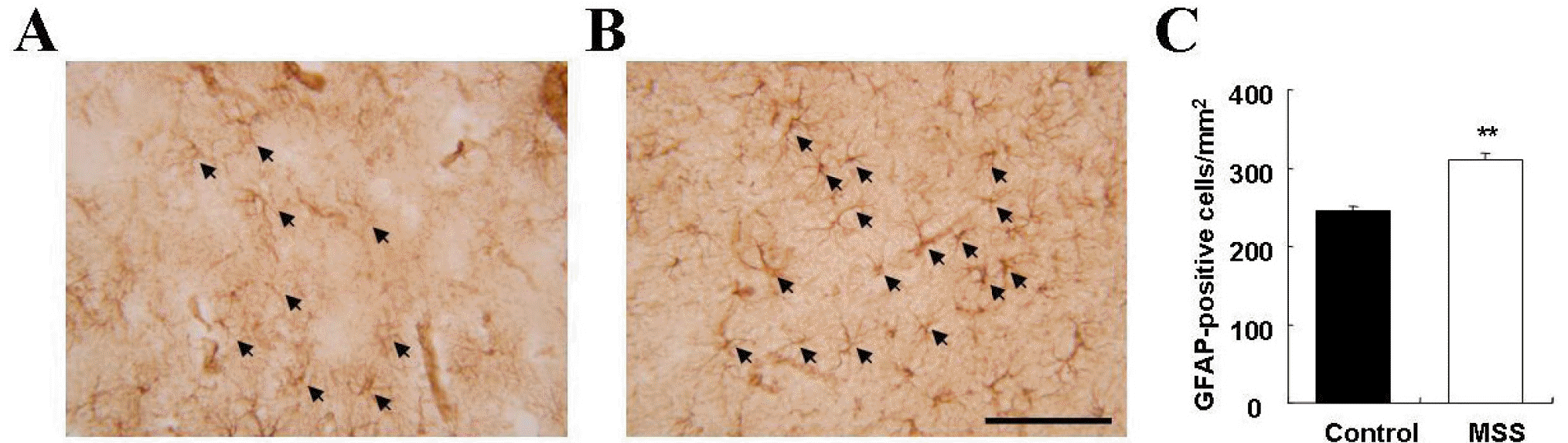
Fig. 4.
Representative photomicrographs of GFAP immunoreactivity in the prefrontal cortex of control (A) and MSS (B) rats. Triple images (0.1 mm2) on the frontal cortex 1 and 2 area were obtained, and density of GFAP-positive cells was calculated (C). Data are expressed as mean±SEM. ∗∗p<0.01, Significant difference from control (Mann-Whitney U-test).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download