Abstract
Skeletal muscle atrophy is a common phenomenon during the prolonged muscle disuse caused by cast immobilization, extended aging states, bed rest, space flight, or other factors. However, the cellular mechanisms of the atrophic process are poorly understood. In this study, we investigated the involvement of mitogen-activated protein kinase (MAPK) in the expression of muscle-specific RING finger 1 (MuRF1) during atrophy of the rat gastrocnemius muscle. Histological analysis revealed that cast immobilization induced the atrophy of the gastrocnemius muscle, with diminution of muscle weight and cross-sectional area after 14 days. Cast immobilization significantly elevated the expression of MuRF1 and the phosphorylation of p38 MAPK. The starvation of L6 rat skeletal myoblasts under serum-free conditions induced the phosphorylation of p38 MAPK and the characteristics typical of cast-immobilized gastrocnemius muscle. The expression of MuRF1 was also elevated in serum-starved L6 myoblasts, but was significantly attenuated by SB203580, an inhibitor of p38 MAPK. Changes in the sizes of L6 myoblasts in response to starvation were also reversed by their transfection with MuRF1 small interfering RNA or treatment with SB203580. From these results, we suggest that the expression of MuRF1 in cast-immobilized atrophy is regulated by p38 MAPK in rat gastrocnemius muscles.
Go to : 
References
Benveniste O., Jacobson L., Farrugia ME., Clover L., Vincent A. MuSK antibody positive myasthenia gravis plasma modifies MuRF-1 expression in C2C12 cultures and mouse muscle in vivo. J Neuroimmunol. 170:41–48. 2005.

Bodine SC., Latres E., Baumhueter S., Lai VK., Nunez L., Clarke BA., Poueymirou WT., Panaro FJ., Na E., Dharmarajan K., Pan ZQ., Valenzuela DM., DeChiara TM., Stitt TN., Yancopoulos GD., Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 294:1704–1708. 2001.

Booth FW., Kelso JR. Production of rat muscle atrophy by cast fixation. Appl Physiol. 34:404–406. 1973.

Booth FW., Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 71:541–585. 1991.

Cai D., Frantz JD., Jr Tawa NE., Melendez PA., Oh BC., Lidov HG., Hasselgren PO., Frontera WR., Lee J., Glass DJ., Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 119:285–298. 2004.
Centner T., Yano J., Kimura E., McElhinny AS., Pelin K., Witt CC., Bang ML., Trombitas K., Granzier H., Gregorio CC., Sorimachi H., Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 306:717–726. 2001.

Freemont PS. RING for destruction? Curr Biol. 10:R84–R87. 2000.
Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 37:1974–1984. 2005.

Gomes MD., Lecker SH., Jagoe RT., Navon A., Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 98:14440–14445. 2001.

Hilder TL., Tou JC., Grindeland RE., Wade CE., Graves LM. Phosphorylation of insulin receptor substrate-1 serine 307 correlates with JNK activity in atrophic skeletal muscle. FEBS Lett. 553:63–67. 2003.

Jagoe RT., Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy. Curr Opin Clin Nutr Metab Care. 4:183–190. 2001.

Kedar V., McDonough H., Arya R., Li HH., Rockman HA., Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA. 101:18135–18140. 2004.

Kyriakis JM., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 81:807–869. 2001.
Lecker SH., Jagoe RT., Gilbert A., Gomes M., Baracos V., Bailey J., Price SR., Mitch WE., Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18:39–51. 2004.

Lee HM., Jeon BH., Won KJ., Lee CK., Park TK., Choi WS., Bae YM., Kim HS., Lee SK., Park SH., Irani K., Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Cir Res. 104:219–227. 2009.
Lee HM., Lee CK., Lee SH., Roh HY., Bae YM., Lee KY., Lim J., Park PJ., Park TK., Lee YL., Won KJ., Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 105:74–81. 2007.

Li YP., Chen Y., John J., Moylan J., Jin B., Mann DL., Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 19:362–370. 2005.
Machida S., Booth FW. Changes in signalling molecule levels in 10-day hindlimb immobilized rat muscles. Acta Physiol Scand. 183:171–179. 2005.

McElhinny AS., Kakinuma K., Sorimachi H., Labeit S., Gregorio CC. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 157:125–136. 2002.

Medina R., Wing SS., Goldberg AL. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 307:631–637. 1995.

Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol. 37:1985–1996. 2005.

Samarel AM., Parmacek MS., Magid NM., Decker RS., Lesch M. Protein synthesis and degradation during starvation-induced cardiac atrophy in rabbits. Cir Res. 60:933–941. 1987.

Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker SH., Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117:399–412. 2004.

Schulze PC., Fang J., Kassik KA., Gannon J., Cupesi M., MacGillivray C., Lee RT., Rosenthal N. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Cir Res. 97:418–426. 2005.

Shi H., Scheffler JM., Zeng C., Pleitner JM., Hannon KM., Grant AL., Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 296:C1040–C1048. 2009.

Skurk C., Izumiya Y., Maatz H., Razeghi P., Shiojima I., Sandri M., Sato K., Zeng L., Schiekofer S., Pimentel D., Lecker S., Taegtmeyer H., Goldberg AL., Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 280:20814–20823. 2005.

Sugita H., Kaneki M., Sugita M., Yasukawa T., Yasuhara S., Martyn JA. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. Am J Physiol Endocrinol Metab. 288:E585–E591. 2005.

Tesseraud S., Métayer-Coustard S., Boussaid S., Crochet S., Audouin E., Derouet M., Seiliez I. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cells. Biochem Biophys Res Commun. 357:181–186. 2007.

Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 26:389–399. 2007.

Tobimatsu K., Noguchi T., Hosooka T., Sakai M., Inagaki K., Matsuki Y., Hiramatsu R., Kasuga M. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun. 378:399–403. 2009.

Wray CJ., Mammen JM., Hershko DD., Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol. 35:698–705. 2003.

Go to : 
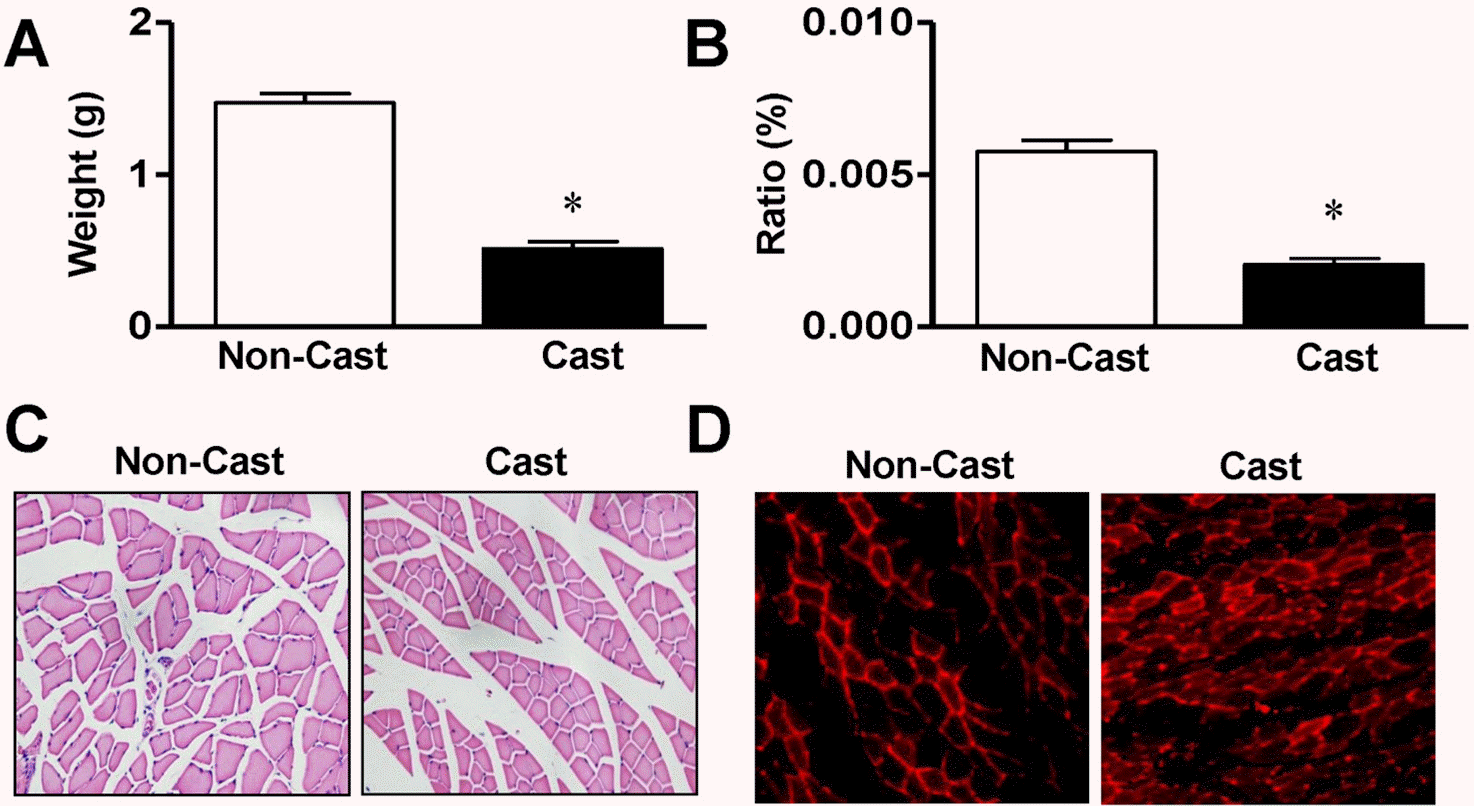 | Fig. 1.Morphological characterization of gastrocnemius muscle from cast-immobilized rat hindlimb. Gastrocnemius muscle weights (A, n=8), ratios of muscle weight to body weight (B, n=8), and cross-sectional areas (C, n=7) were measured 14 days after cast immobilization. The muscle fibers were visualized with H&E staining, as described in the Materials and methods. (D) Immunohistological analysis of rats at day 14 after cast immobilization. MuRF1 expression (red) was visualized with a phase-contrast microscope (×400). Representative result of six independent experiments. ∗Significantly different from the noncasted control (p<0.05). |
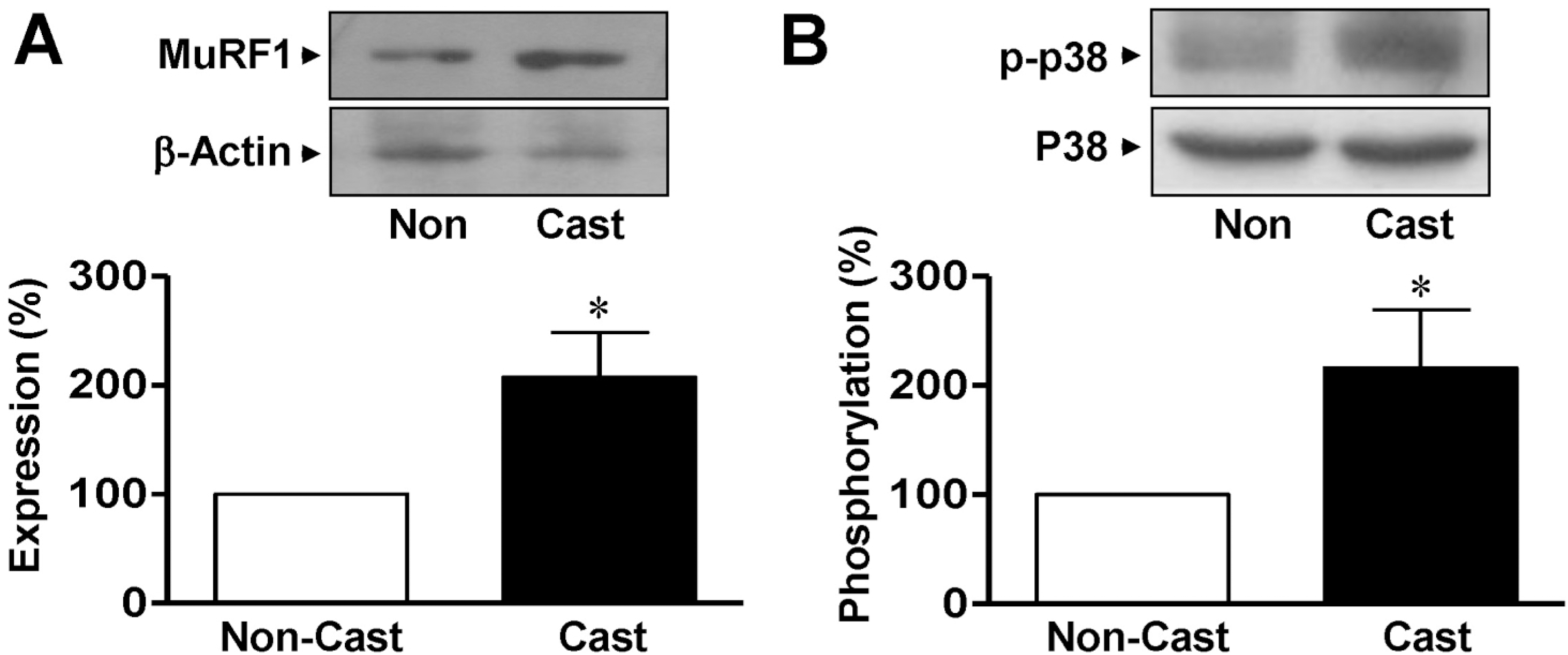 | Fig. 2.Expression of MuRF1 and p38 MAPK in gastrocnemius muscles from cast-immobilized rats. Immunoblotting analysis of MuRF1 (A) and p38 MAPK (B) in the gastrocnemius muscle. Protein expression and phosphorylation were examined using anti-nonphospho- and anti-phosphospecific antibodies, respectively. The statistical results were obtained from the upper panels. The levels of MuRF1 expression and p38 MAPK phosphorylation in the noncasted muscle group were considered to be 100% (n=4). ∗Significantly different from the non-casted controls (p<0.05). Non, non-casted. |
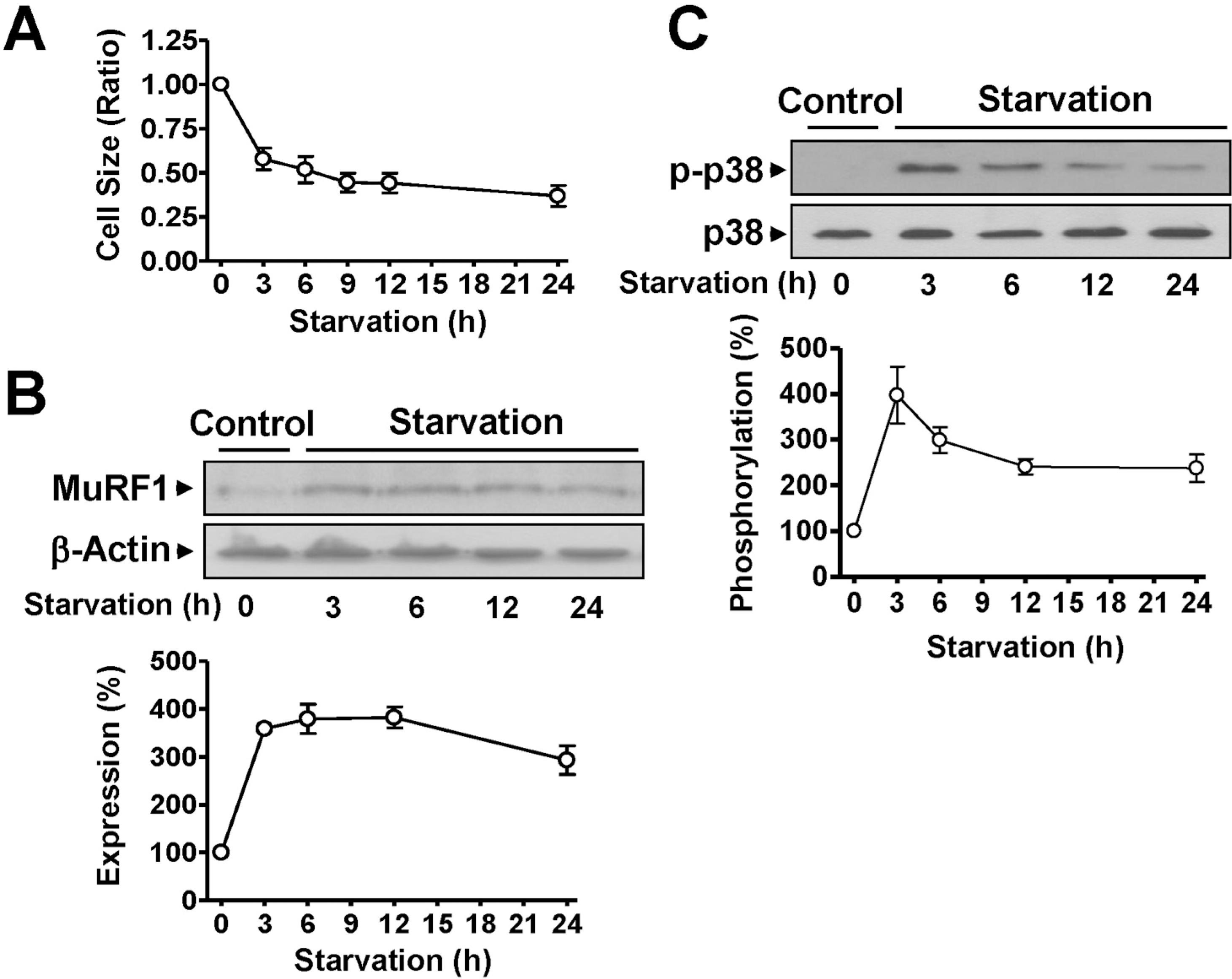 | Fig. 3.Changes in the characteristics of rat L6 skeletal myoblasts starved in serum-free medium. (A) Changes in the cell size. The cells were cultured in serum-free medium for the indicated times and visualized with H&E staining. The cell sizes were analyzed under a phase-contrast microscope (×100, n=8). (B) The expression of MuRF1. The cells were starved in serum-free medium for 24 h and the lysates were subjected to immuno-blotting analysis with anti-MuRF1 and anti-β-actin antibodies. The statistical results were obtained from the upper panel. (C) p38 MAPK phosphorylation in cells starved in serumfree medium. The phosphorylation and expression of p38 AMPK were examined using anti-phospho and anti-nonphospho antibodies, respectively. The basal level of phosphorylation in nonstarved control cells was considered to be 100% (n=5). |
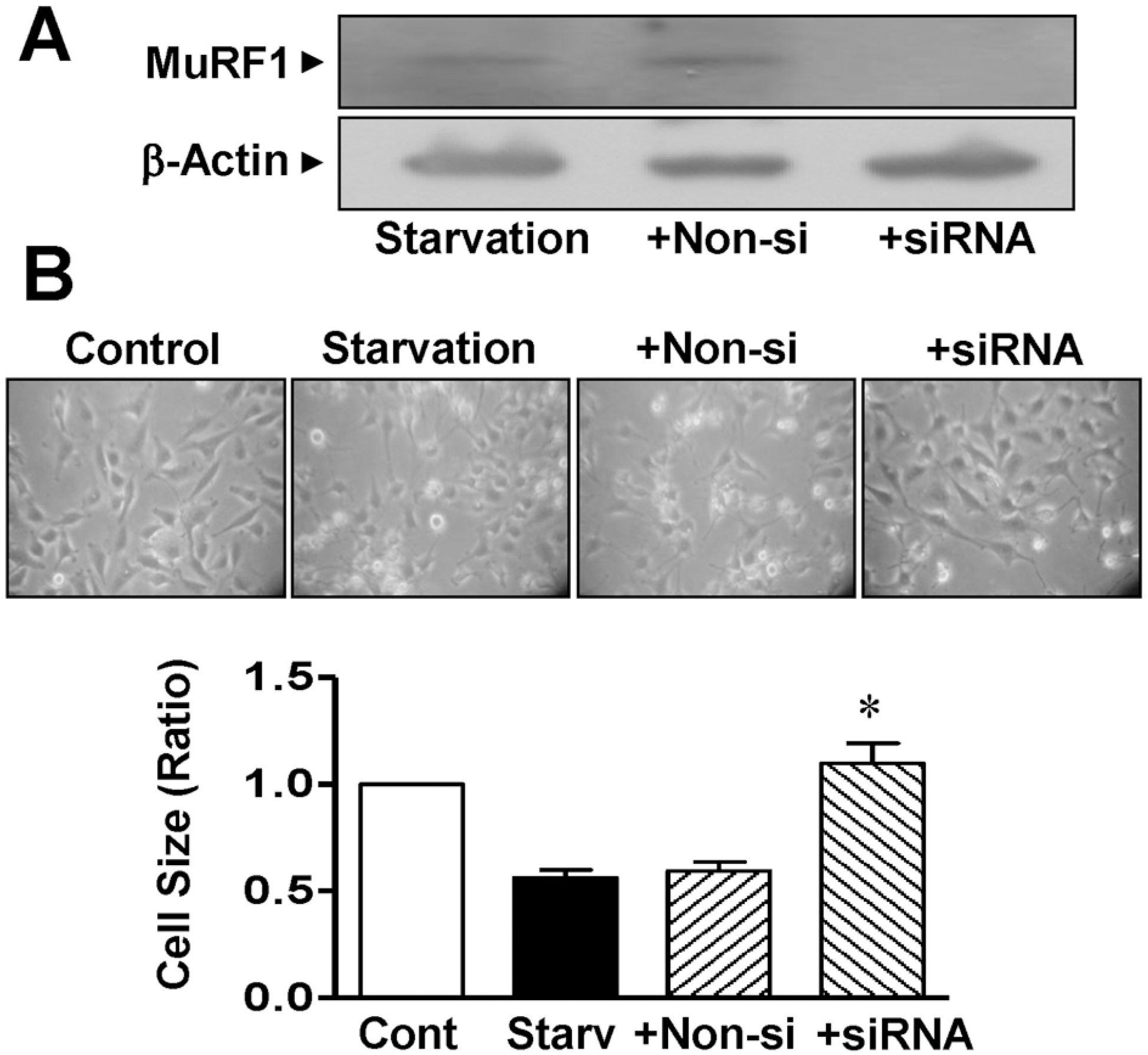 | Fig. 4.Changes in the size of L6 myoblasts knocked down with MuRF1 siRNA. (A) MuRF1 knockdown using MuRF1 siRNA. The cells were transfected with MuRF1 siRNA. Extracts from the cells were immunoblotted with anti-MuRF1 and anti-β-actin antibodies (n=4). (B) Changes in cell size caused by transfection with MuRF1 siRNA. After transfection of the cells with MuRF1 siRNA, the cell sizes were measured under a phase-contrast microscope (×100). ∗Significant differences between siRNA-treated cells and nonsilenced controls (n=8; p<0.05). Control, nonstarved control cells; Starv, starvation; + Non-si, nonsilencing; + siRNA, MuRF1 siRNA. |
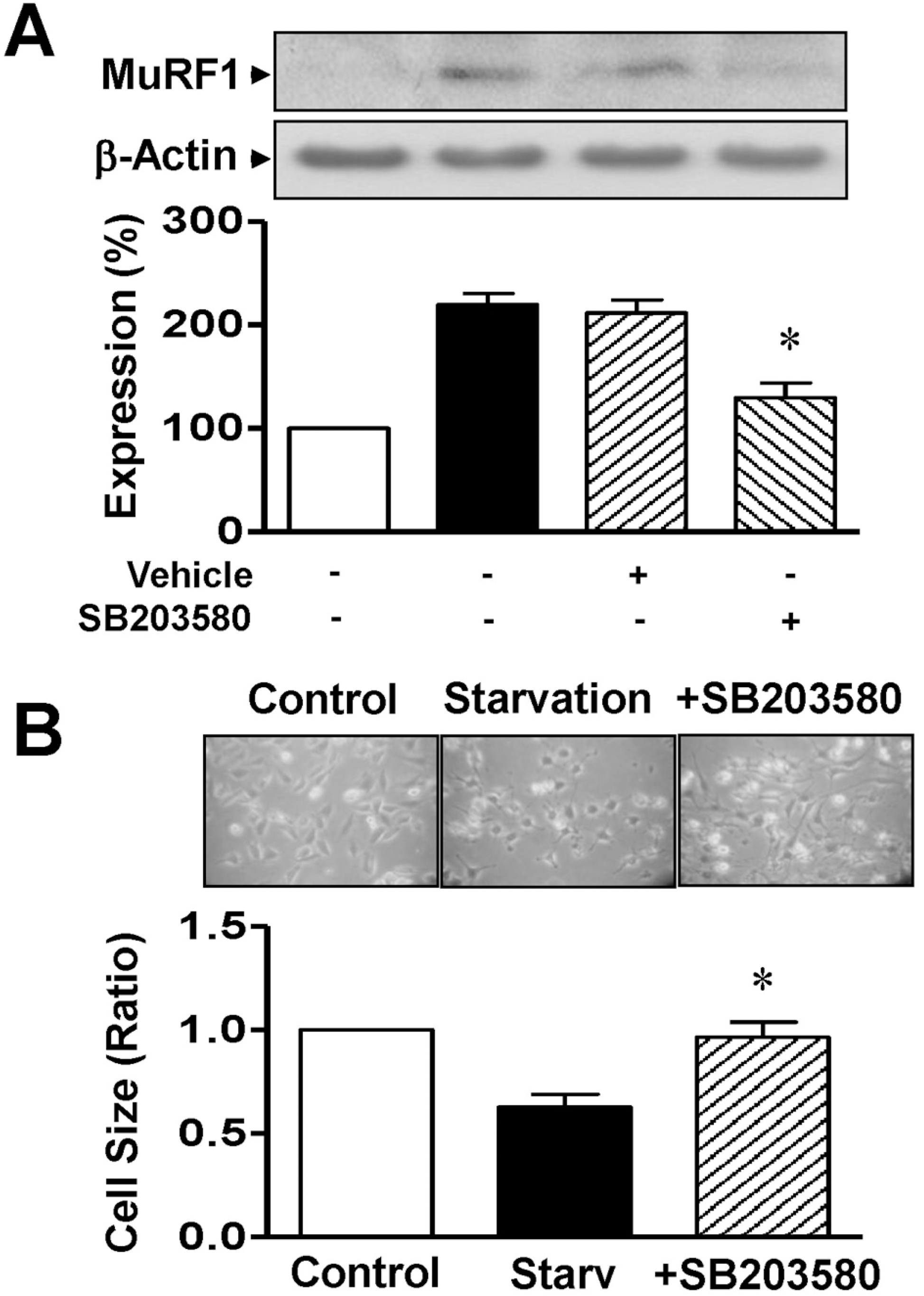 | Fig. 5.Effects of p38 MAPK on MuRF1 expression and cell size in serum-starved L6 myoblasts. (A) Effect of p38 MAPK inhibitor on MuRF1 expression. Cells were starved for 12 h in serum-free medium with 0.1% DMSO or 10 μM SB203580. Cell lysates were immunoblotted with anti-MuRF1 and anti-β-actin antibodies. The statistical results were obtained from the upper panel. MuRF1 expression in nonstarved cells was considered to be 100% (n=4). (B) Effect of p38 MAPK inhibitor on cell size. The cells were starved for 12 h in serum-free medium with 0.1% DMSO or 10 μM SB203580 and the cell sizes were measured under a phase-contrast microscope (×100; n=8). ∗Significant differences between the starved and nonstarved control groups (p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download