Abstract
Despite the potential importance of the human regulator of calcineurin 1 (RCAN-1) gene in the modulation of cell survival under stress, little is known about its role in death-inducing signal pathways. In this study, we addressed the effects of RCAN1.4 knockdown on cellular susceptibility to apoptosis and the activation of death pathway proteins. Transfection of siRNAs against RCAN1.4 resulted in enhanced Fas- and etoposide-induced apoptosis, which was associated with increased expression and translocation of Bax to mitochondria. Our results suggest that enhanced expression and activation of p53 was responsible for the upregulation of Bax and the increased sensitivity to apoptosis, which could be reversed by p53 knockdown. To explain the observed upregulation of p53, we propose a downregulation of the ubiquitin ligase HDM2, probably translationally. These findings show the importance of appropriate RCAN1.4 expression in the modulation of cell survival and reveal a link between RCAN1.4 and p53.
References
Banin S., Moyal L., Shieh S., Taya Y., Anderson CW., Chessa L., Smorodinsky NI., Prives C., Reiss Y., Shiloh Y., Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 281:1674–1677. 1998.

Barak Y., Juven T., Haffner R., Oren M. Mdm2 expression is induced by wild type p53 activity. EMBO J. 12:461–468. 1993.

Canman CE., Lim DS., Cimprich KA., Taya Y., Tamai K., Sakaguchi K., Appella E., Kastan MB., Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 281:1677–1679. 1998.

De Benedetti A., Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 23:3189–3199. 2004.

Ermak G., Harris CD., Davies KJ. The DSCR1 (Adapt78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium-mediated stress damage, including transient oxidative stress. FASEB J. 16:814–824. 2002.
Ermak G., Morgan TE., Davies KJ. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease. J Biol Chem. 276:38787–38794. 2001.

Fang S., Jensen JP., Ludwig RL., Vousden KH., Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 275:8945–8951. 2000.

Fuentes JJ., Genesca L., Kingsbury TJ., Cunningham KW., Perez-Riba M., Estivill X., de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 9:1681–1690. 2000.

Fuentes JJ., Pritchard MA., Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 44:358–361. 1997.
Fuentes JJ., Pritchard MA., Planas AM., Bosch A., Ferrer I., Estivill X. A new human gene from the Down syndrome critical region encodes a proline-rich protein highly expressed in fetal brain and heart. Hum Mol Genet. 4:1935–1944. 1995.

Harris CD., Ermak G., Davies KJ. Multiple roles of the DSCR1 (Adapt78 or RCAN1) gene and its protein product calcipressin 1 (or RCAN1) in disease. Cell Mol Life Sci. 62:2477–2486. 2005.

Head E., Lott IT., Patterson D., Doran E., Haier RJ. Possible compensatory events in adult Down syndrome brain prior to the development of Alzheimer disease neuropathology: targets for nonpharmacological intervention. J Alzheimers Dis. 11:61–76. 2007.

Hsu YT., Wolter KG., Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 94:3668–3672. 1997.

Kubbutat MH., Jones SN., Vousden KH. Regulation of p53 stability by Mdm2. Nature. 387:299–303. 1997.

Kubbutat MH., Ludwig RL., Ashcroft M., Vousden KH. Regulation of Mdm2-directed degradation by the C terminus of p53. Mol Cell Biol. 18:5690–5698. 1998.

Lee HJ., Kim YS., Sato Y., Cho YJ. RCAN1–4 knockdown attenuates cell growth through the inhibition of Ras signaling. FEBS Lett. 583:2557–2564. 2009.

Miyashita T., Krajewski S., Krajewska M., Wang HG., Lin HK., Liebermann DA., Hoffman B., Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 9:1799–1805. 1994.
Porta S., Serra SA., Huch M., Valverde MA., Llorens F., Estivill X., Arbones ML., Marti E. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration. Hum Mol Genet. 16:1039–1050. 2007.

Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell. 95:5–8. 1998.
Rothermel B., Vega RB., Yang J., Wu H., Bassel-Duby R., Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 275:8719–8725. 2000.

Ryeom S., Baek KH., Rioth MJ., Lynch RC., Zaslavsky A., Birsner A., Yoon SS., McKeon F. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 13:420–431. 2008.

Ryeom S., Greenwald RJ., Sharpe AH., McKeon F. The threshold pattern of calcineurin-dependent gene expression is altered by loss of the endogenous inhibitor calcipressin. Nat Immunol. 4:874–881. 2003.

Sanna B., Brandt EB., Kaiser RA., Pfluger P., Witt SA., Kimball TR., van Rooij E., De Windt LJ., Rothenberg ME., Tschop MH., Benoit SC., Molkentin JD. Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc Natl Acad Sci U S A. 103:7327–7332. 2006.

Wolter KG., Hsu YT., Smith CL., Nechushtan A., Xi XG., Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.

Fig. 1.
RCAN1.4 knockdown enhances Fas-antibody- or etoposide-mediated apoptosis in U87MG cells. (A) Cells were transfected with siRNA against RCAN1.4 (siRCAN259) or negative control siRNA (siCon). After 48 h, cells were treated with an activating anti-Fas antibody (CH11) for 5 h to induce apoptosis. Cells were then washed, stained with Annexin V, and analyzed by FACS. (B) Cells transfected with two doses of siRCAN259 (20 and 40 pmol/well in a 6-well plate) were treated with activating anti-Fas antibody and then subjected to immunoblot analysis for the indicated proteins. (C) Cells transfected with the indicated siRNAs were treated with activating anti-Fas antibody for 6 h or etoposide for 16 h. The cells were then subjected to a caspase activity assay (Promega) according to the manufacturer's instructions.
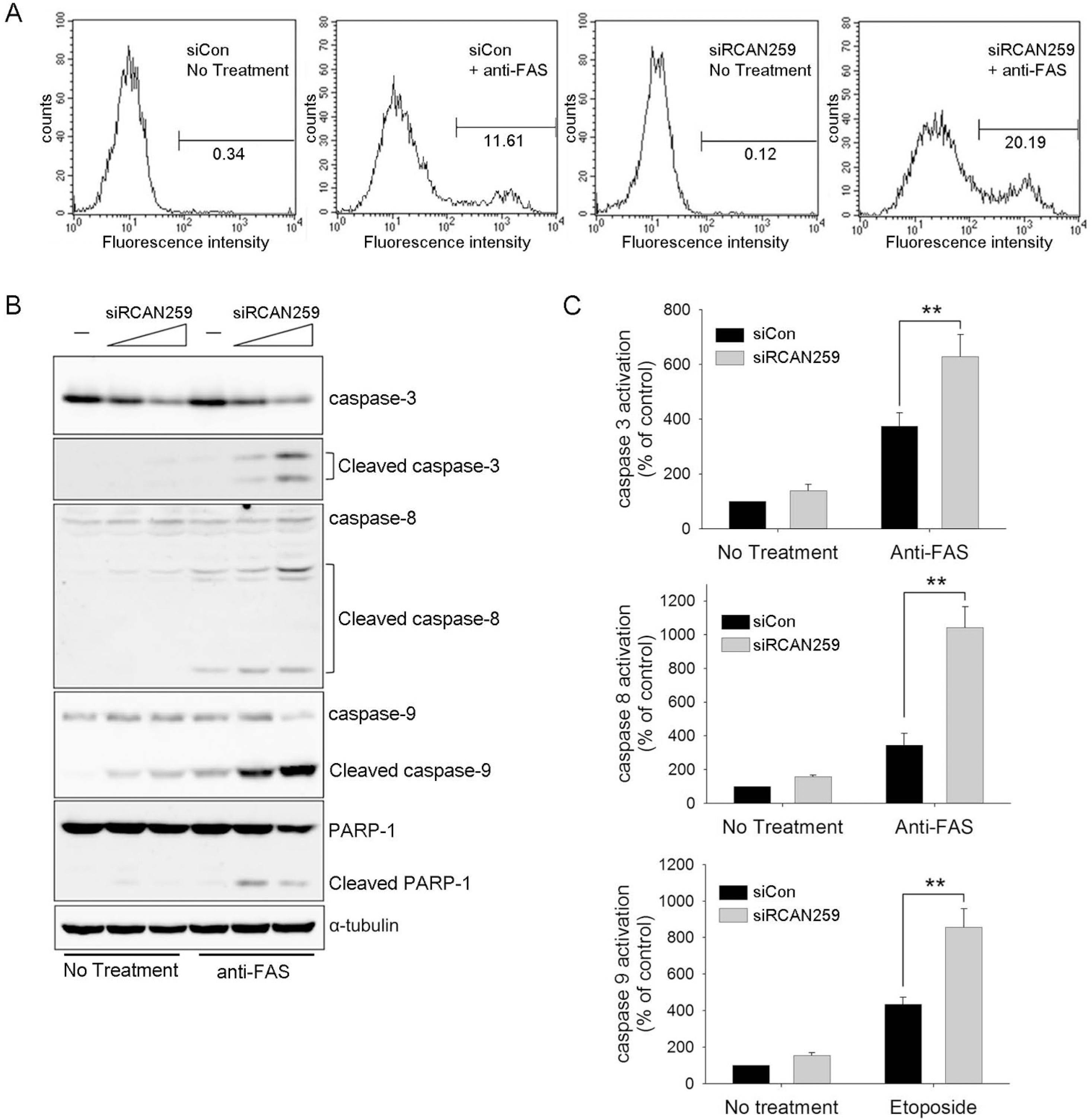
Fig. 2.
RCAN1.4 knockdown enhances mitochondria-mediated apoptosis. Forty-eight hours after transfection with siRCAN259, siRCAN1348, or siCon, cells were treated with activating anti-Fas antibody for 6 h or etoposide for 16 h. After cells were disrupted as indicated in the methods section, the cytosol fractions (A) or the mitochondrial fractions (B) were subjected to immunoblot analysis for the indicated proteins.
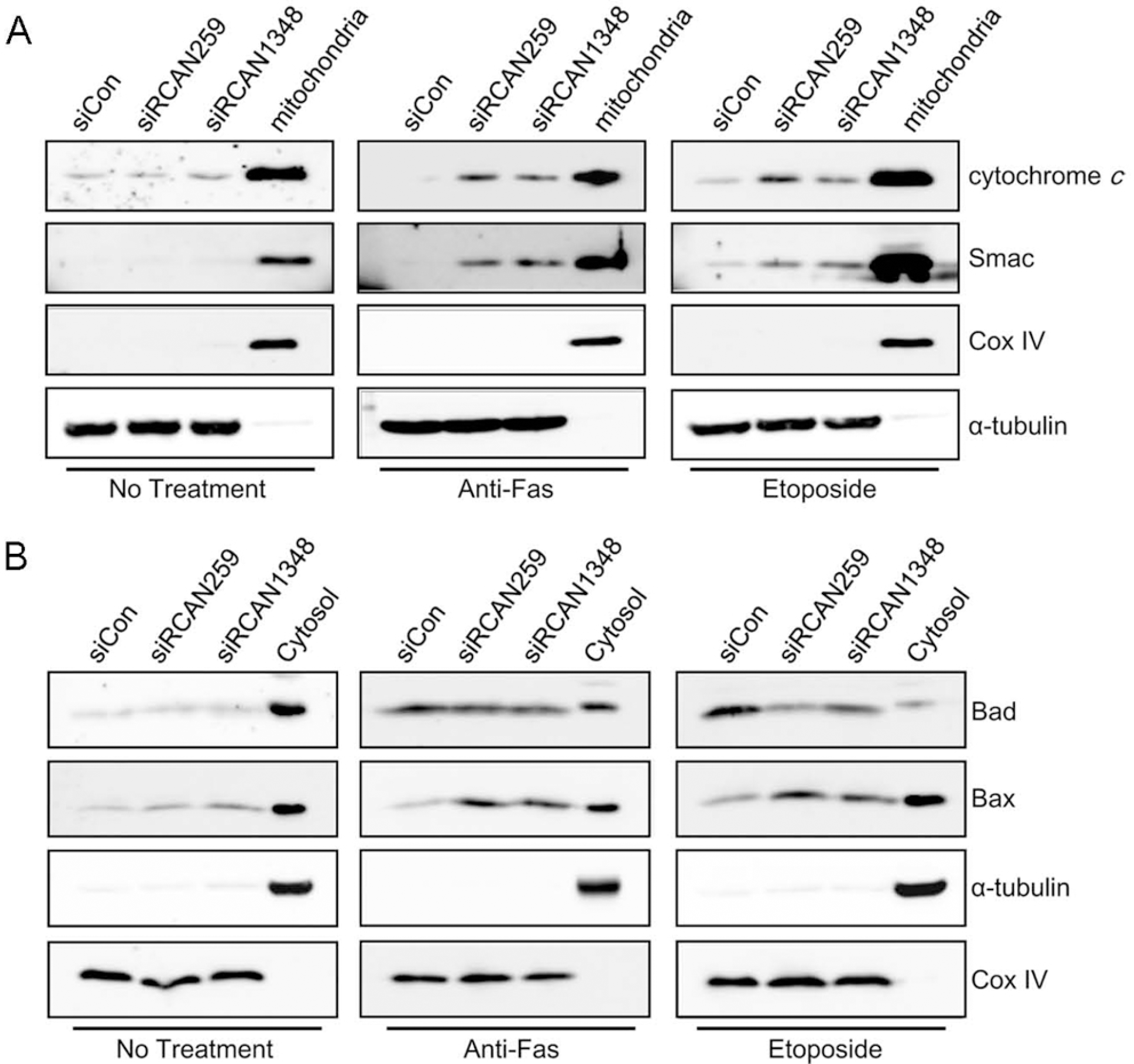
Fig. 3.
RCAN1.4 knockdown upregulates Bax and p53 expression. (A) Forty-eight hours after transfection with the indicated siRNAs, cells were treated with activating anti-Fas antibody for 6 h or etoposide for 16 h. Whole lysates from the cells were subjected to immunoblot analysis for the indicated proteins. (B) Twenty-four hours after transfection with the indicated siRNAs, cells were transfected with a vector containing p53-Luc. Twenty-four hours after the final transfection, luciferase activities were measured. The results are presented as the average±SD (Student's t test; ∗∗p<0.001). (C) Forty-eight hours after transfection with the indicated siRNAs, whole lysates from the cells were subjected to immunoblot analysis for the indicated proteins. (D) Forty-eight hours after transfection with the indicated siRNAs, cells were treated with etoposide for 16 h. Whole lysates from the cells were subjected to immunoblot analysis for the indicated proteins.
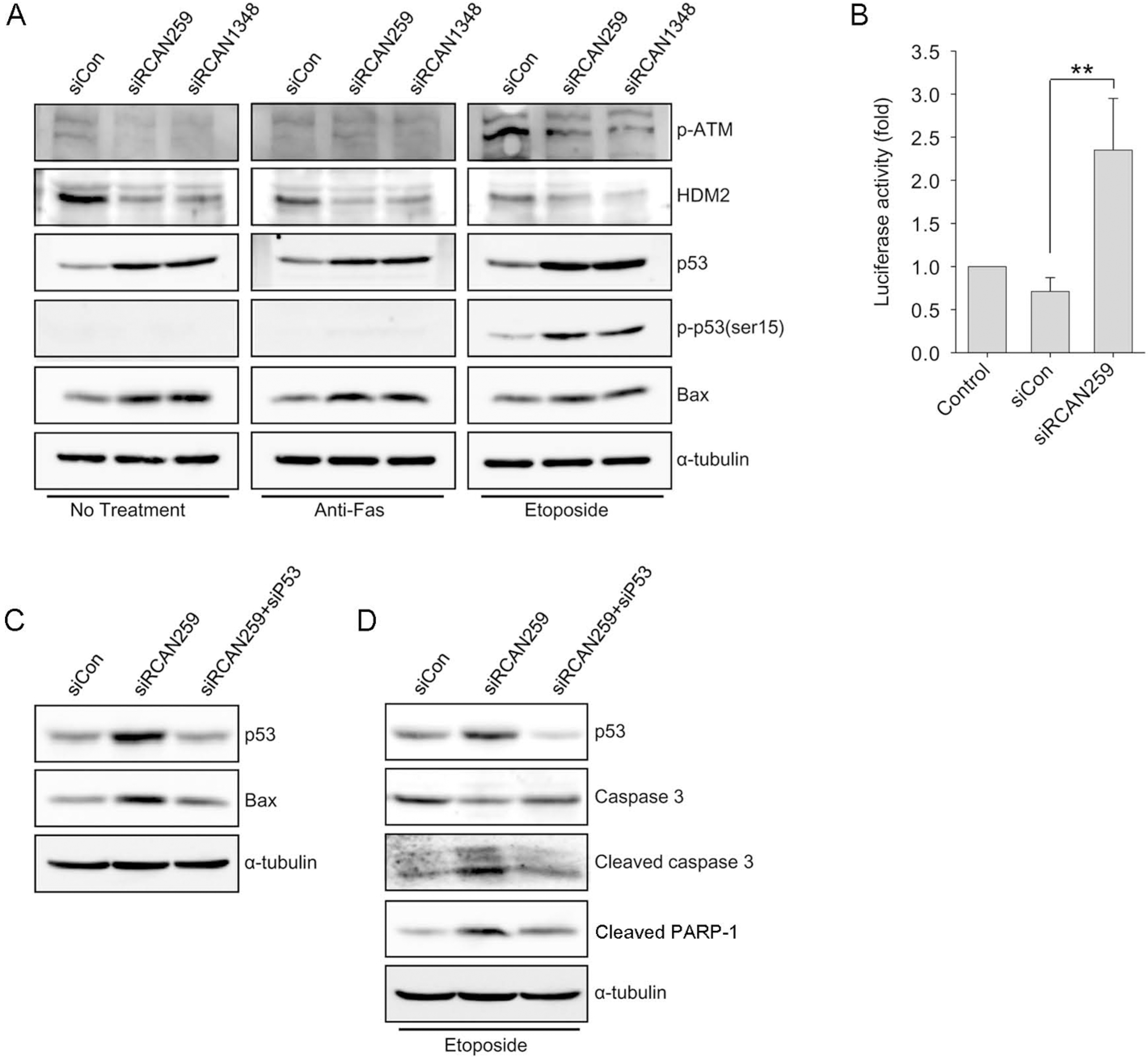
Fig. 4.
RCAN1.4 knockdown increases p53 expression while decreasing HDM2 expression. (A) Forty-eight hours after transfection with three doses of siRCAN259 (5, 20, or 40 pmol/well in a 6-well plate), whole lysates from the cells were subjected to immunoblot analysis for the indicated proteins. (B, C) Forty-eight hours after transfection with siRCAN259, p53 and HDM2 transcript levels were measured by real-time RT-PCR. (D) Twenty-four hours after transfection with siRCAN259 or siCon, cells were transfected with a vector containing wild-type (wt-HDM2) or mutants (C464A and 440Δ) of HDM2. Twenty-four hours after the final transfection, whole lysates from the cells were subjected to immunoblot analysis with an antibody specific to HDM2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download