Abstract
Rifampicin is a macrocyclic antibiotic which is used extensively for treatment against Mycobacterium tuberculosis and other mycobacterial infections. Recently, a number of studies have focused on the immune-regulatory effects of rifampicin. Therefore, we hypothesized that rifampicin may influence the TLR2 expression in LPS-activated RAW 264.7 cells. In this study, we determined that rifampicin suppresses LPS-induced TLR2 mRNA expression. The down-regulation of TLR2 expression coincided with decreased production of TNF-α. Since NF-κB is a major transcription factor that regulates genes for TLR2 and TNF-α, we examined the effect of rifampicin on the LPS-induced NF-κB activation. Rifampicin inhibited NF-κB DNA-binding activity in LPS-activated RAW 264.7 cells, while it did not affect IKK α/β activity. However, rifampicin slightly inhibited the nuclear translocation of NF-κB p65. In addition, rifampicin increased physical interaction between pregnane X receptor, a receptor for rifampicin, and NF-κB p65, suggesting pregnane X receptor interferes with NF-κB binding to DNA. Taken together, our results demonstrate that rifampicin inhibits LPS-induced TLR2 expression, at least in part, via the suppression of NF-κB DNA-binding activity in RAW 264.7 cells. Thus, the present results suggest that the rifampicin-mediated inhibition of TLR2 via the suppression of NF-κB DNA-binding activity may be a novel mechanism of the immune-suppressive effects of rifampicin.
Go to : 
References
Aravalli RN., Hu S., Rowen TN., Palmquist JM., Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 175:4189–4193. 2005.

Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 14:649–683. 1996.
Bellahsene A., Forsgren A. Effect of rifampin on the immune response in mice. Infect Immunl. 27:15–20. 1980.
Ghosh S., May MJ., Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 16:225–260. 1998.
Gu X., Ke S., Liu D., Sheng T., Thomas PE., Rabson AB., Gallo MA., Xie W., Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 281:17882–17889. 2006.
Haehnel V., Schwarzfischer L., Fenton MJ., Rehli M. Transcriptional regulation of the human toll-like receptor 2 gene in monocytes and macrophages. J Immunol. 168:5629–5637. 2002.
Jeon YJ., Han SH., Lee YW., Lee M., Yang KH., Kim HM. Dexamethasone inhibits IL-1 beta gene expression in LPS-stimulated RAW 264.7 cells by blocking NF-kappa B/Rel and AP-1 activation. Immunopharmacology. 48:173–183. 2000.
Kalkhoven E., Wissink S., van der Saag PT., van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 271:6217–6224. 1996.
Lehmann JM., McKee DD., Watson MA., Willson TM., Moore JT., Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 102:1016–1023. 1998.

Li T., Chen W., Chiang JY. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J Lipid Res. 48:373–384. 2007.

Li T., Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 288:G74–G84. 2005.
Mlambo G., Sigola LB. Rifampicin and dexamethasone have similar effects on macrophage phagocytosis of zymosan, but differ in their effects on nitrite and TNF-alpha production. Int Immunopharmacol. 3:513–522. 2003.
Moreau A., Vilarem MJ., Maurel P., Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm. 5:35–41. 2008.

Mun HS., Aosai F., Norose K., Chen M., Piao LX., Takeuchi O., Akira S., Ishikura H., Yano A. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 15:1081–1087. 2003.

Nakamura K., Moore R., Negishi M., Sueyoshi T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 282:9768–9776. 2007.

Pahlevan AA., Wright DJ., Bradley L., Smith C., Foxwell BM. Potential of rifamides to inhibit TNF-induced NF-kappaB activation. J Antimicrob Chemother. 49:531–534. 2002.

Palvimo JJ., Reinikainen P., Ikonen T., Kallio PJ., Moilanen A., Janne OA. Mutual transcriptional interference between RelA and androgen receptor. J Biol Chem. 271:24151–24156. 1996.

Ray A., Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 91:752–756. 1994.

Ray A., Prefontaine KE., Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 269:12940–12946. 1994.

Shah YM., Ma X., Morimura K., Kim I., Gonzalez FJ. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 292:G1114–G1122. 2007.
Stein B., Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 15:4971–4979. 1995.

Xu DX., Wei W., Sun MF., Wu CY., Wang JP., Wei LZ., Zhou CF. Kupffer cells and reactive oxygen species partially mediate lipopolysaccharide-induced downregulation of nuclear receptor pregnane × receptor and its target gene CYP3a in mouse liver. Free Radic Biol Med. 37:10–22. 2004.
Yuhas Y., Berent E., Ovadiah H., Azoulay I., Ashkenazi S. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 50:396–398. 2006.

Zhang H., LeCulyse E., Liu L., Hu M., Matoney L., Zhu W., Yan B. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 368:14–22. 1999.

Zhou C., Tabb MM., Nelson EL., Grun F., Verma S., Sadatrafiei A., Lin M., Mallick S., Forman BM., Thummel KE., Blumberg B. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 116:2280–2289. 2006.
Go to : 
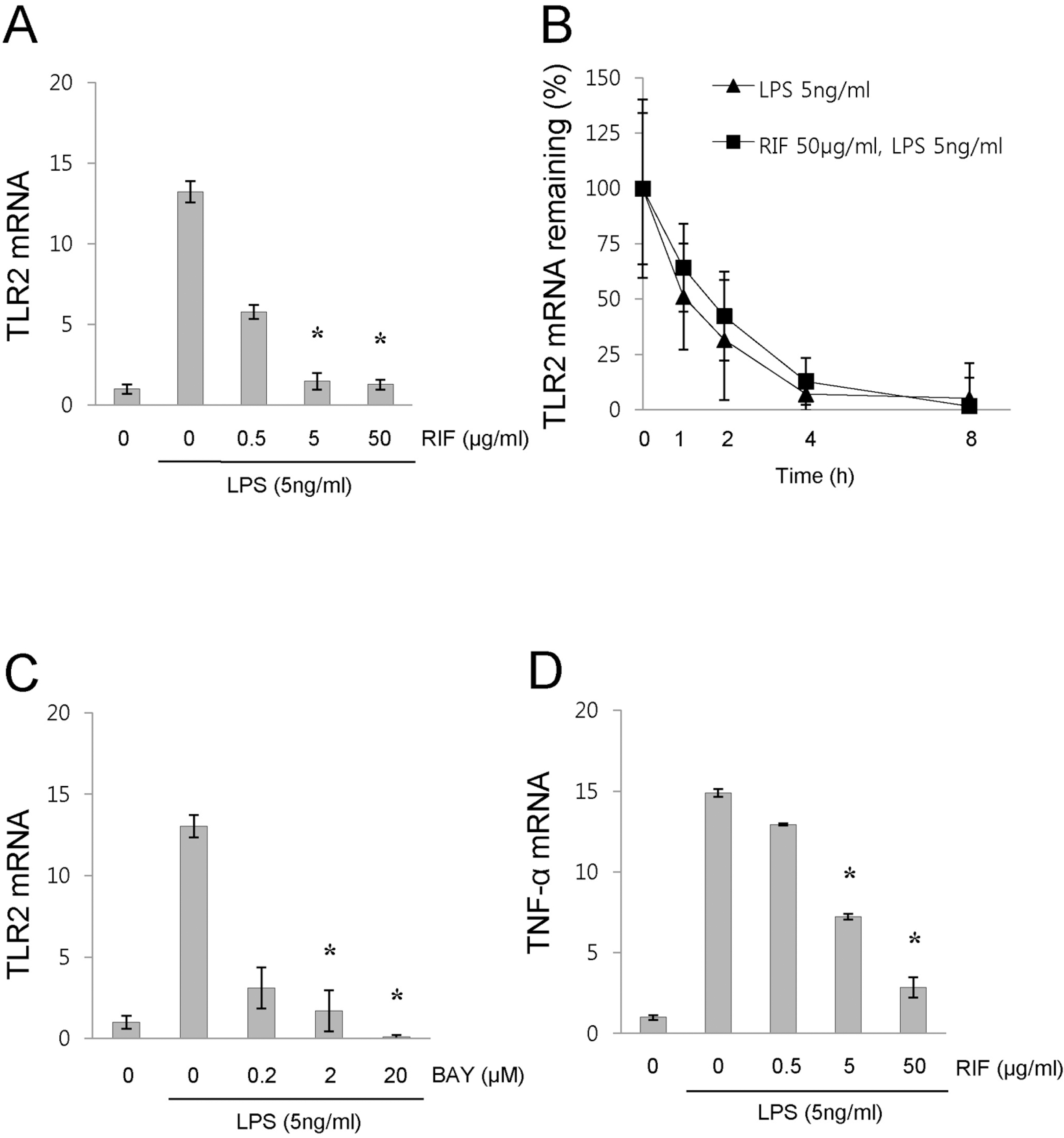 | Fig. 1.Rifampicin inhibits the LPS-induced expression of TLR2 mRNA, and the expression of TLR2 mRNA is dependent on the NF-κB pathway. (A, D) RAW 264.7 cells were pretreated with rifampicin at the indicated concentrations or with DMSO for 1 h and then stimulated with LPS (5 ng/ml) for 4 hours. mRNA levels of TLR2 (A) and TNF-α (D) were determined by quantitative real time RT-PCR. (B) For TLR2 mRNA stability assay, RAW 264.7 cells were pre-treated with rifampicin (50 μg/ml) and then treated with LPS (5 ng/ml) for 2 hours before adding actinomycin D1 (5 μg/ml). At designated times, the level of TLR2 mRNA was determined by quantitative real time-RT-PCR. (C) RAW 264.7 cells were pretreated with BAY 11–7085, an inhibitor of NF-κB, at the indicated concentrations or with DMSO for 1 h and then stimulated with LPS (5 ng/ml) for 4 hours. mRNA levels of TLR2 were determined by quantitative real time RT-PCR. The results are shown as the means±SD of data from at least three separate experiments, each performed with triplicate samples. ∗p<0.01 versus non-treated control cells. RIF, rifampicin. |
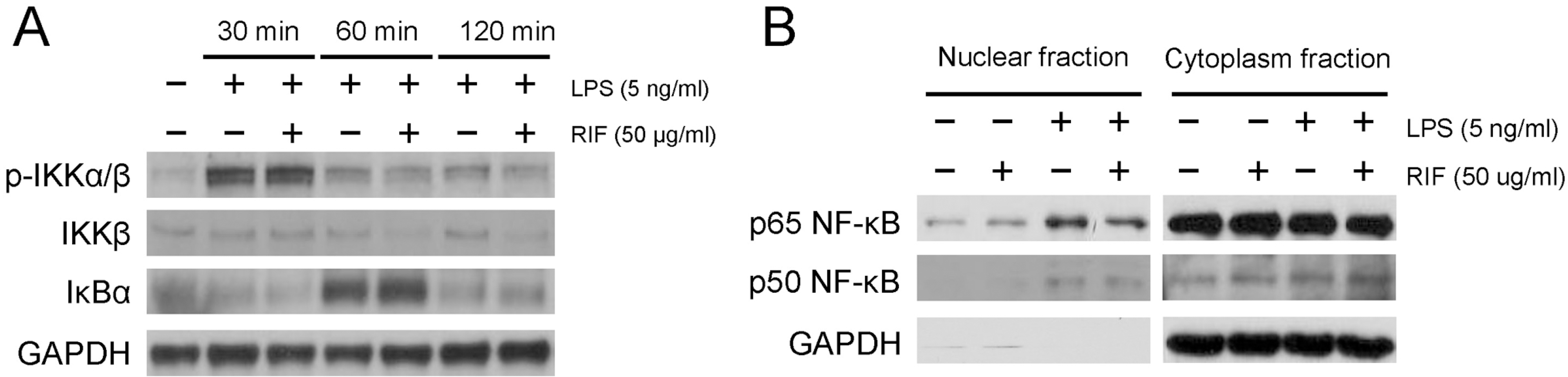 | Fig. 2.Rifampicin inhibits the nuclear translocation of NF-κB p65 in RAW 264.7 cells. RAW 264.7 cells were pre-treated with rifampicin (50 μg/ml) or with DMSO for 1 h and then stimulated with LPS (5 ng/ml) for the indicated times. (A) The levels of phosphorylation of IKKα/β and the expression of IκBα were determined by immunoblot analyses. (B) The nuclear translocation of NF-κB subunits p50 and p65 were determined by immunoblot analyses. After treatment, nuclear and cytoplasmic extracts were prepared using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Inc.). Similar results were observed in three independent experiments. RIF, rifampicin. |
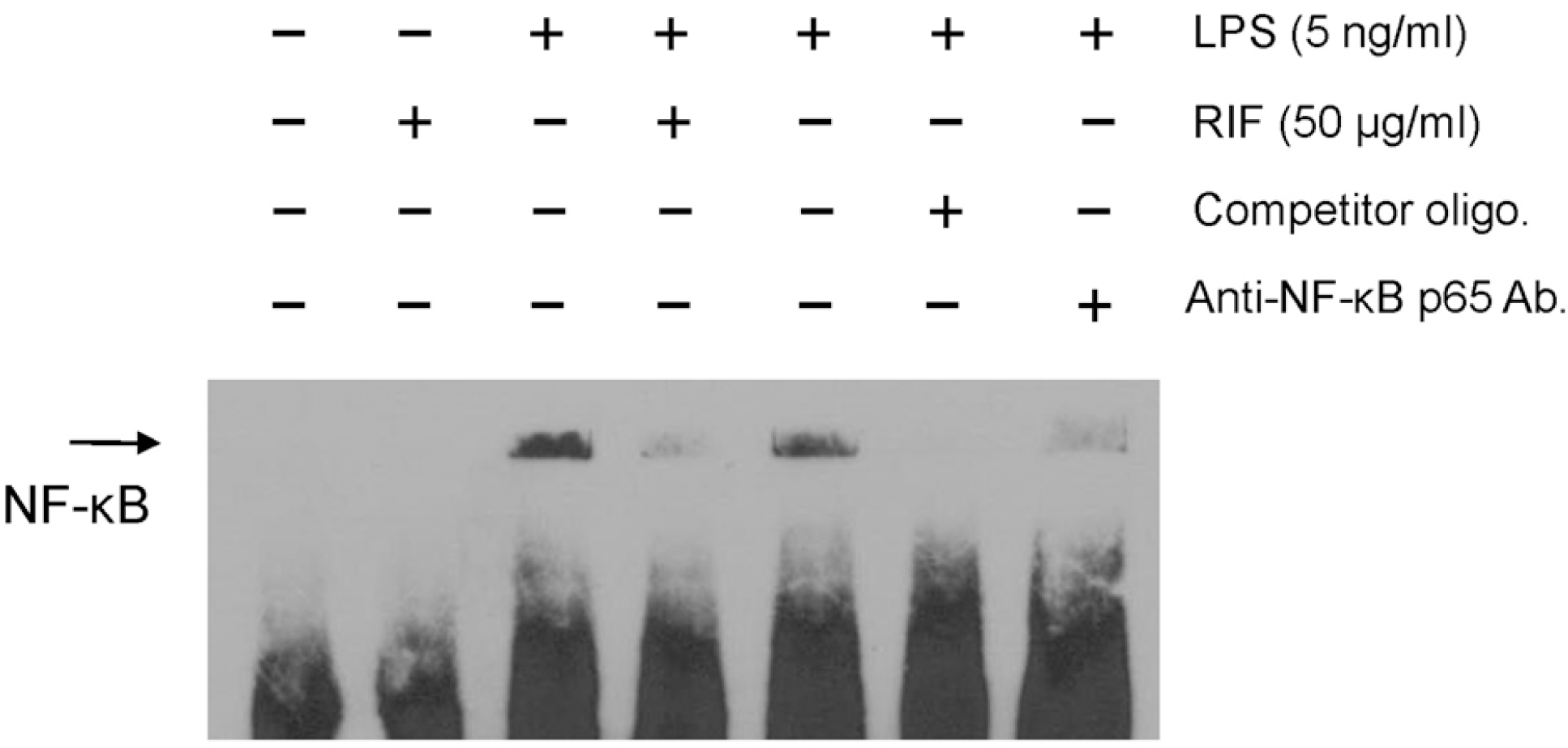 | Fig. 3.Rifampicin suppresses LPS-induced NF-κB DNA binding activity in RAW 264.7 cells. RAW 264.7 cells were pre-treated with rifampicin (50 μg/ml) or with DMSO for 1 h and then stimulated with LPS (5 ng/ml) for 1 h. DNA binding activity of NF-κB subunits was analyzed by EMSA. After treatment, nuclear extracts were prepared using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Inc.). For the competition assay, a 200-fold excess of unlabeled probe was added together with the labeled probe. For the supershift assay, 1 μg of antibody against NF-κB p65 was added together with the nuclear extract. Similar results were observed in three independent experiments. RIF, rifampicin; Competitor oligo., unlabeled oligonucleotides probe for NF-κB p65. |
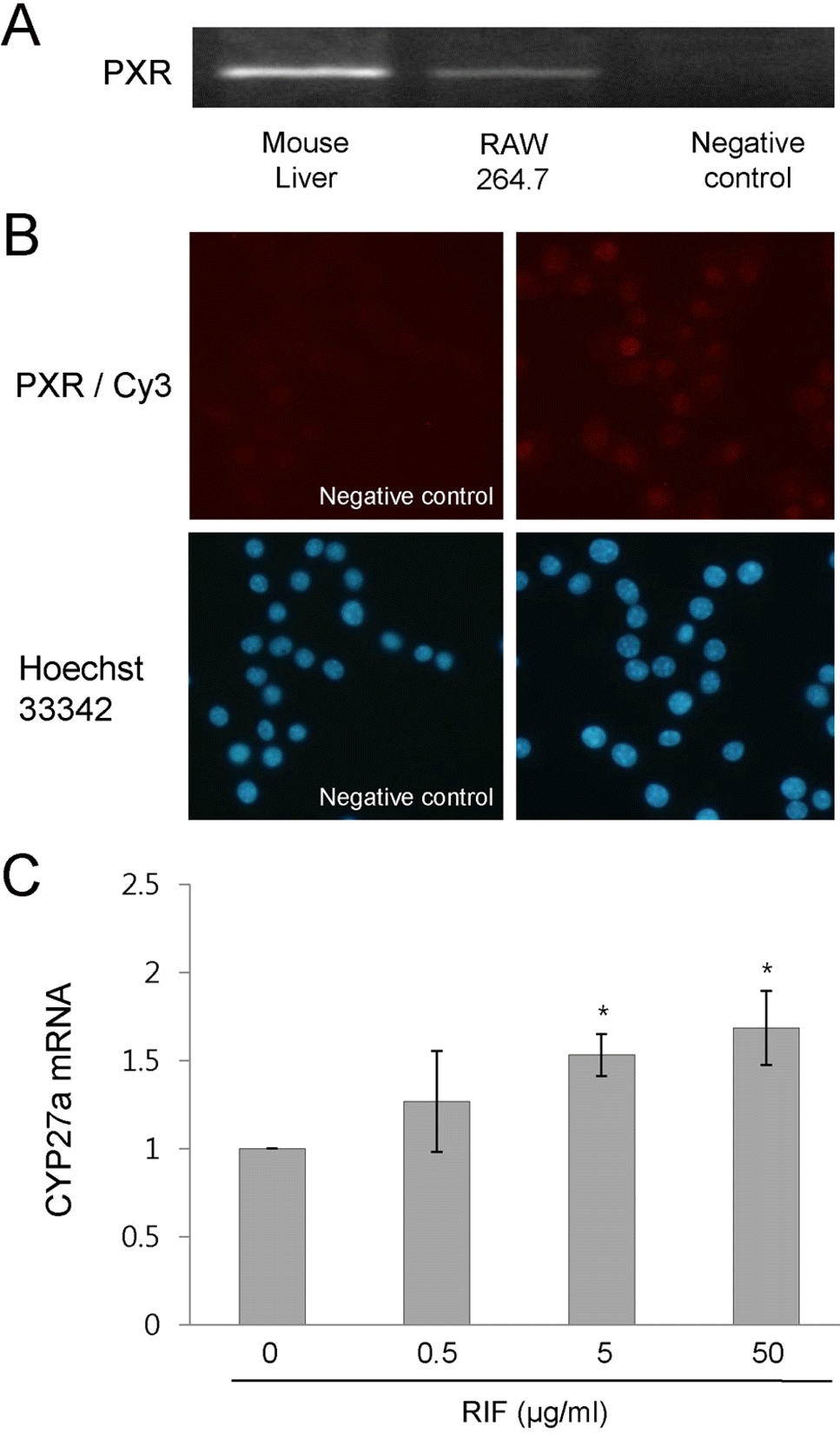 | Fig. 4.Rifampicin activates PXR in RAW 264.7 cells. (A) PXR mRNA expression was determined by RT-PCR. (B) The expression pattern of PXR was determined by immunostaining. The cells were incubated overnight with an anti-PXR antibody (Santa Cruz Biotechnology) at 4°C. After washing with PBS, cells were incubated with the corresponding Cy3-conjugated secondary IgG at room temperature for 2 h. Nuclei were counterstained for 15 min with 10 μM Hoechst 33342 (Sigma-Aldrich Co. Ltd). (C) RAW 264.7 cells were treated with rifampicin at the indicated concentrations or with DMSO for 4 h. The expression of CYP27A1 mRNA was determined by quantitative real time RT-PCR. Similar results were observed in three independent experiments. ∗p<0.01 versus non-treated control cells. PXR, pregnane X receptor; PXR/Cy3, anti-PXR antibody/Cy3-conjugated secondary antibody; RIF, rifampicin. |
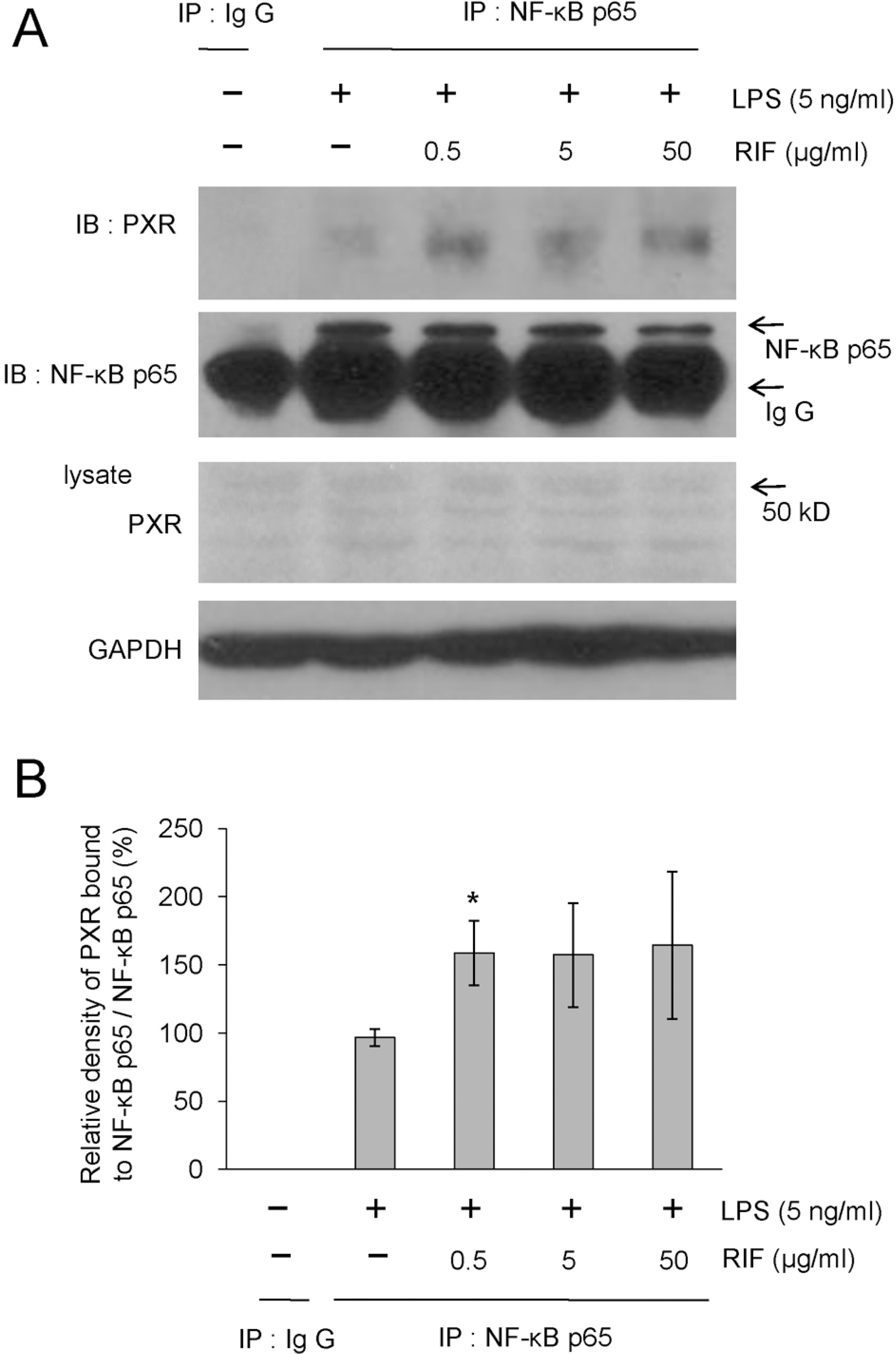 | Fig. 5.Rifampicin increases the physical association of PXR with NF-κB p65 in LPS-activated RAW 264.7 cells. (A, B) RAW 264.7 cells were pre-treated with rifampicin at the indicated concentrations or with DMSO for 1 h and then stimulated with LPS (5 ng/ml) for 1 h. The physical association between PXR and NF-κB subunits was examined by a co-immunoprecipitation assay. The cell lysates were immunoprecipitated with an irrelevant rabbit IgG as a negative control and antibody against NF-κB p65, together with protein A Sepharose™ CL-4B (Amersham Biosciences, Uppsla, Sweden). The immunoprecipitates were then electrophoresed on SDS-PAGE gels, transferred onto PVDF membranes (Millipore, Bedford, MA), and immunoblotted with an antibody against PXR and NF-κB p65. Similar results were observed in three independent experiments (A). The results are shown as the means±SD of data from at least three separate experiments, each performed with triplicate samples. ∗p<0.05 versus the values for the LPS treatment only (B). RIF, rifampicin. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download