Abstract
The auditory cortex (A1) encodes the acquired significance of sound for the perception and interpretation of sound. Nitric oxide (NO) is a gas molecule with free radical properties that functions as a transmitter molecule and can alter neural activity without direct synaptic connections. We used whole-cell recordings under voltage clamp to investigate the effect of NO on spontaneous GABAergic synaptic transmission in mechanically isolated rat auditory cortical neurons preserving functional presynaptic nerve terminals. GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs) in the A1 were completely blocked by bicuculline. The NO donor, S-nitroso-N-acetylpenicillamine (SNAP), reduced the GABAergic sIPSC frequency without affecting the mean current amplitude. The SNAP-induced inhibition of sIPSC frequency was mimicked by 8-bromoguanosine cyclic 3′,5′-monophosphate, a membrane permeable cyclic-GMP analogue, and blocked by 2-(4-carboxyphenyl)-4,4,5,5-tetramethy-limidazoline-1-oxyl-3-oxide, a specific NO scavenger. Blockade of presynaptic K+ channels by 4-aminopyridine, a K+ channel blocker, increased the frequencies of GABAergic sIPSCs, but did not affect the inhibitory effects of SNAP. However, blocking of presynaptic Ca2+ channels by Cd2+, a general voltage-dependent Ca2+ channel blocker, decreased the frequencies of GABAergic sIPSCs, and blocked SNAP-induced reduction of sIPSC frequency. These findings suggest that NO inhibits spontaneous GABA release by activation of cGMP-dependent signaling and inhibition of presynaptic Ca2+ channels in the presynaptic nerve terminals of A1 neurons.
References
Ahern GP., Klyachko VA., Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 25:510–517. 2002.

Akaike N., Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 44:433–473. 1994.

Arnold WP., Mittal CK., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 74:3203–3207. 1977.

Boulton CL., Irving AJ., Southam E., Potier B., Garthwaite J., Collingridge GL. The nitric oxide-cyclic GMP pathway and synaptic transmission in rat hippocampal slices. Eur J Neurosci. 6:1528–1535. 1994.
Bredt DS., Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 86:9030–9033. 1989.

Caspary DM., Ling L., Turner JG., Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 211:1781–1791. 2008.

Caspary DM., Raza A., Lawhorn Armour BA., Pippen J., Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implication for neural presbycusis. J Neurosci. 10:2363–2372. 1990.
Chik CL., Liu QY., Li B., Karspinski E., Ho AK. cGMP inhibits L-type Ca2+ channel currents through protein phosphorylation in rat pinealocytes. J Neurosci. 15:3104–3109. 1995.
Grassi C., D'Ascenzo M., Valente A., Battista Azzena G. Ca2+ channel inhibition induced by nitric oxide in rat insulinoma RINm5F cells. Pflügers Arch. 437:241–247. 1999.
Hendry SH., Schwark HD., Jones EG., Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 7:1503–1519. 1987.

Huh YB., Park DC., Yeo SG., Cha CI. Evidence for increased NADPH-diaphorase-positive neurons in the central auditory system of the aged rat. Acta Otolaryngol. 128:648–653. 2008.

Hupfer K., Jurgens U., Ploog D. The effect of superior temporal lesions on the recognition of species-specific calls in the squirrel monkey. Exp Brain Res. 30:75–87. 1977.

Jang IS., Rhee JS., Watanabe T., Akaike N., Akaike N. Histaminergic modulation of GABAergic transmission in rat ventromedial hypothalamic neurones. J Physiol (Lond.). 534:791–803. 2001.

Klyachko VA., Ahern GP., Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron. 31:1015–1025. 2001.

Knight JA. The process and theories of aging. Annu Clin Lab Sci. 25:1–12. 1995.
Ko GY., Kelly PT. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 19:6784–6794. 1999.

Kraus MM., Prast H. Involvement of nitric oxide, cyclic GMP and phosphodiesterase 5 in excitatory amino acid and GABA release in the nucleus accumbens evoked by activation of the hippocampal fimbria. Neuroscience. 112:331–343. 2002.

Lee JJ., Cho YW., Huh YB., Cha CI., Yeo SG. Effect of nitric oxide on auditory cortical neurons of aged rats. Neurosci Lett. 447:37–41. 2008.

Liang L., Lu T., Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol. 87:2237–2261. 2002.

Li DP., Chen SR., Finnegan TF., Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 554:100–110. 2004.

Ling LL., Hughes LF., Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 132:1103–1113. 2005.

Manzoni O., Prezeau L., Marin P., Deshager S., Bockaert J., Fagni L. Nitric oxide induced blockade of NMDA receptors. Neuron. 8:653–662. 1992.
Neff WD. The brain and hearing: auditory discriminations affected by brain leisions. Ann Otol Rhinol Laryngol. 86:500–506. 1977.
Ozaki M., Shibuya I., Kabashima N., Isse T., Noguchi J., Ueta Y., Inoue Y., Shigematsu A., Yamashita H. Preferential potentiation by nitric oxide of spontaneous inhibitory postsynaptic currents in rat supraoptic neurons. J Neuroendocrinol. 12:273–281. 2000.
Peinado M. Histology and histochemistry of the aging cerebral cortex; an overview. Microsc Res Tech. 43:1–7. 1998.

Pineda J., Kogan JH., Aghajanian GK. Nitric oxide and carbon monoxide activate locus coeruleus neurons through a cGMP-dependent protein kinase: involvement of a nonselective cationic channel. J Neurosci. 16:1389–1399. 1996.

Prieto JJ., Peterson BA., Winer JA. Morphology and spatial distribution of GABAergic neurons in cat primary auditory cortex. J Comp Neurol. 344:349–382. 1994.
Raza A., Arneric SP., Milbrandt J., Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear Res. 77:221–230. 1994.

Schreiner CE., Read HL., Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci. 23:501–529. 2000.

Stamler JS., Toone EJ., Lipton SA., Sucher NJ. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 18:691–696. 1997.

Tewari K., Simard JM. Sodium nitroprusside and cGMP decrease Ca2+ availability in basilar artery smooth muscle cells. Pflügers Arch. 433:304–311. 1997.
Tohse N., Sperelakis N. cGMP inhibits the activity of single calcium channels in embryonic chick heart cells. Circ Res. 69:325–331. 1991.

Wei JY., Ethan J., Cohen D., Daw NW., Barnstable CJ. cGMP-induced presynaptic depression and postsynaptic facilitation at glutamatergic synapses in visual cortex. Brain Res. 927:42–54. 2002.

Wu LG., Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. J Neurosci. 14:5613–5622. 1994.
Fig. 1.
GABAergic sIPSCs recorded from mechanically dissociated A1 neurons. (A) A representative trace of sIPSCs recorded before, during, and after the application of 50 μM bicuculline at a VH of –60 mV. The external solution contained 300 nM TTX, 3 μM CNQX and 10 μM AP5. Insets represent typical traces with an expanded time scale. (B) Traces of sIPSCs recorded at VH of –60, –40, 0, and +40 mV. Intracellular and extracellular Cl– concentrations were 140 mM and 161 mM, respectively. I-V curve for the mean amplitude of sIPSCs recorded at various VHs. Each point is the mean of four neurons.
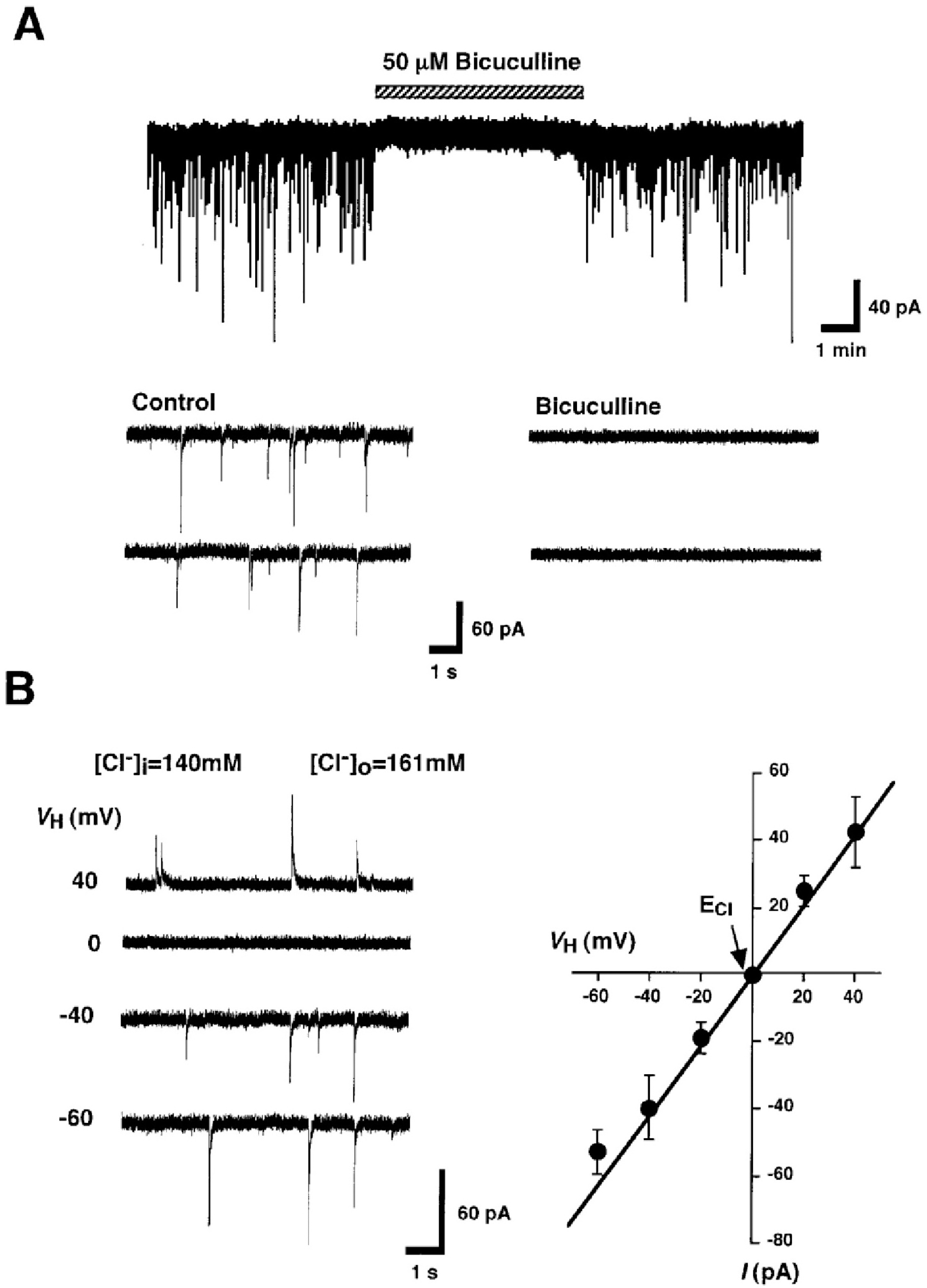
Fig. 2.
SNAP decreases GABAergic sIPSCs by presynaptic mechanisms. (A) A representative trace of sIPSCs recorded before, during, and after the application of 100 μM SNAP. Insets, represent typical traces with an expanded time scale (lower) and SNAP-induced changes in the rate of rise and decay time constant (upper). (B) Cumulative probability distributions for inter-event interval (left) and amplitude (right) of GABAergic sIPSCs recorded from the same neuron. p values indicate the results of K-S tests for frequency and amplitude. (C) Each column is the mean of 20 neurons. All frequencies and amplitudes are normalized to those of control sIPSCs. Asterisks represent a statistically significant difference (∗p<0.05, paired two-tailed t-test).
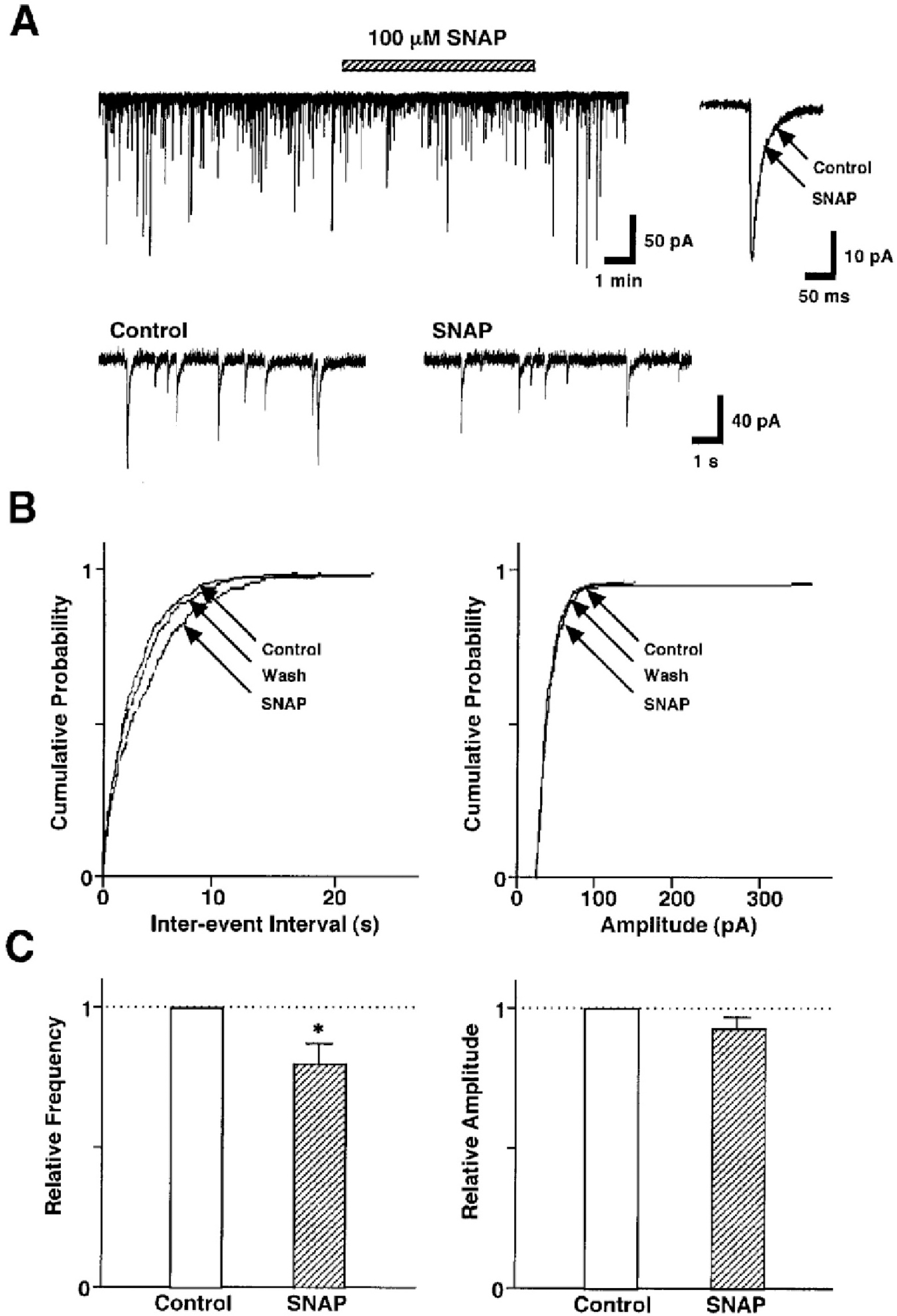
Fig. 3.
SNAP-induced presynaptic inhibition of GABAergic sIPSCs is coupled to the cGMP-dependent signal transduction pathway. (A) A representative trace of sIPSCs recorded before, during, and after the application of 100 μM 8-Br-cGMP. Insets, represent typical traces with an expanded time scale (lower) and 8-Br-cGMP-induced changes in the rate of rise and decay time constant (upper). (B) Cumulative probability distributions for inter-event interval (left) and amplitude (right) of GABAergic sIPSCs recorded from the same neuron. p values indicate the results of K-S tests for frequency and amplitude. (C) Each column is the mean of 6 neurons. All frequencies and amplitudes are normalized to those of control sIPSCs. Asterisks represent a statistically significant difference (∗p<0.05, paired two-tailed t-test).
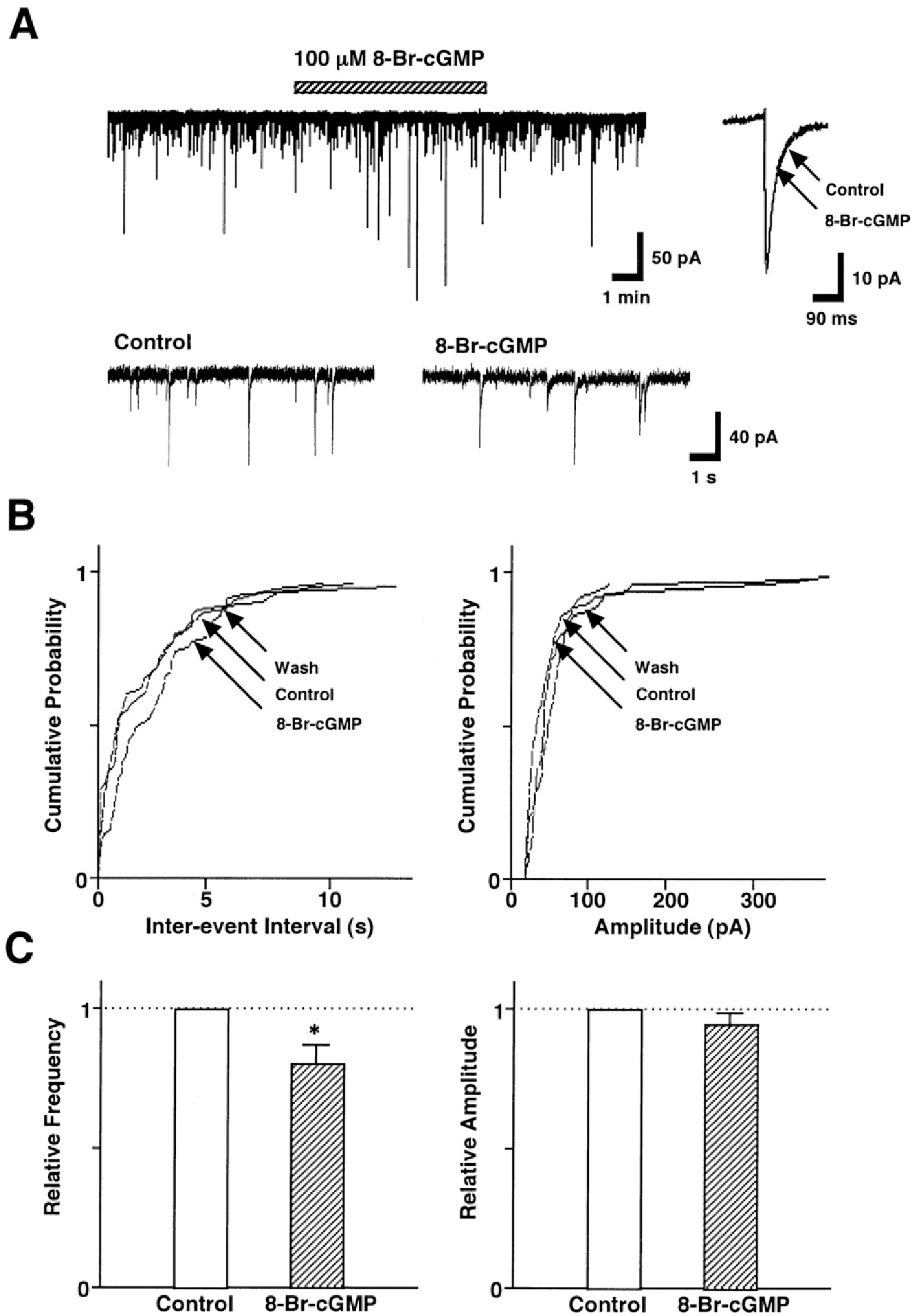
Fig. 4.
Effect of carboxy-PTIO on the SNAP-induced inhibition of GABAergic sIPSCs. (A) Representative recording traces of sIPSCs observed before and during the application of 100 μM SNAP in the absence or presence of 1 μM carboxy-PTIO. Insets represent typical traces with an expanded time scale. (B) Cumulative distributions for inter-event interval (left) and amplitude (right) of sIPSCs recorded from the same neuron. p values indicate the results of K-S tests for frequency and amplitude. (C) Each column is the mean of 5 neurons. All frequencies and amplitudes are normalized to those of control sIPSCs.
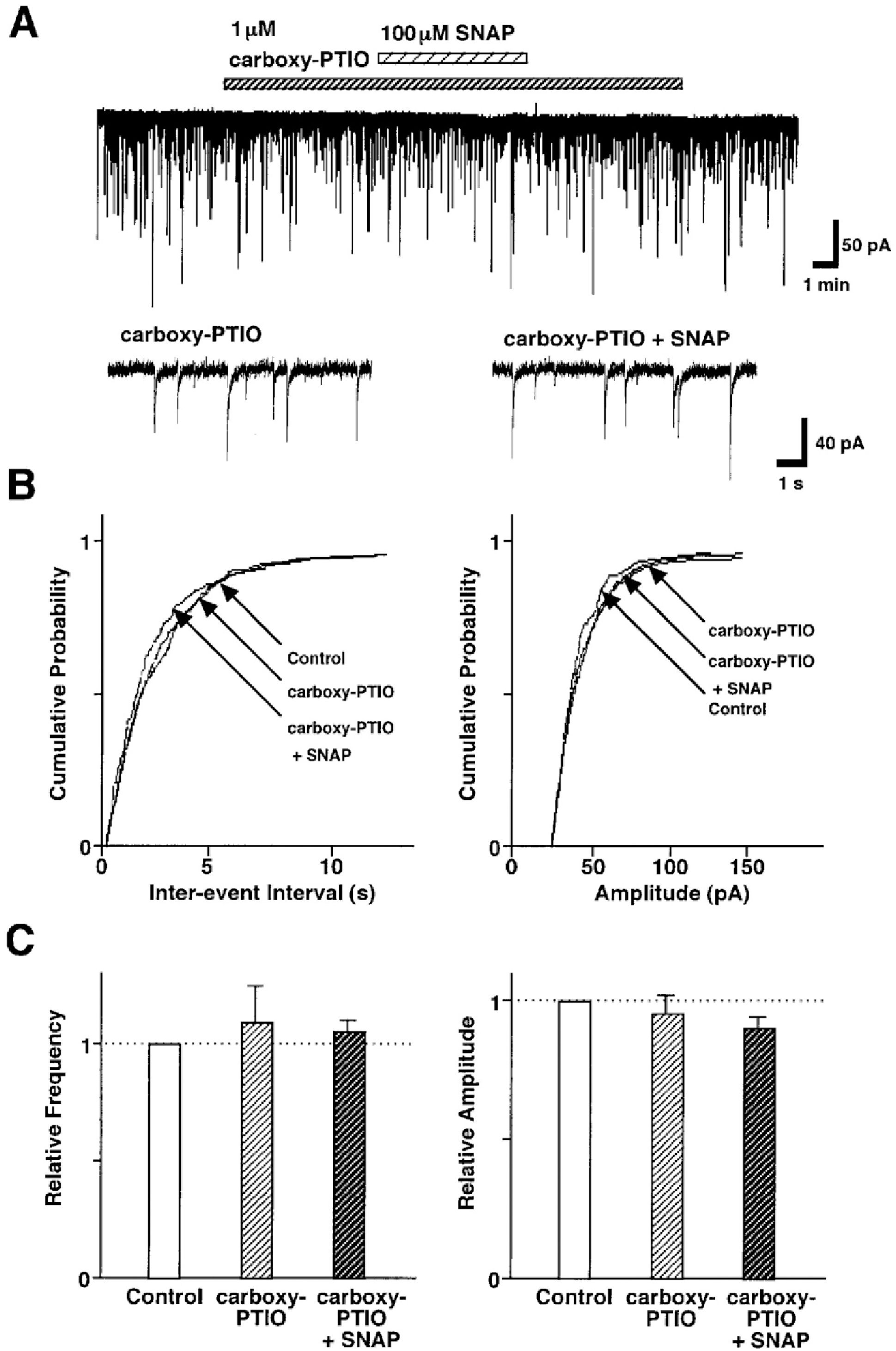
Fig. 5.
Effect of the 4-aminopyridine on the SNAP-induced inhibition of GABAergic sIPSCs. (A) Representative recording traces of sIPSCs show the change in the SNAP effect in the presence of 100 μM 4-AP, a K+ channel blocker. Insets represent typical traces with an expanded time scale. (B) Cumulative distributions for inter-event interval (left) and amplitude (right) of sIPSCs in the same neuron. p values indicate the results of K-S tests for frequency and amplitude. (C) All amplitudes and frequencies are normalized to the control sIPSCs. Each column is the mean of 9 neurons. Asterisks represent a statistically significant difference (∗p<0.05, paired two-tailed t-test).
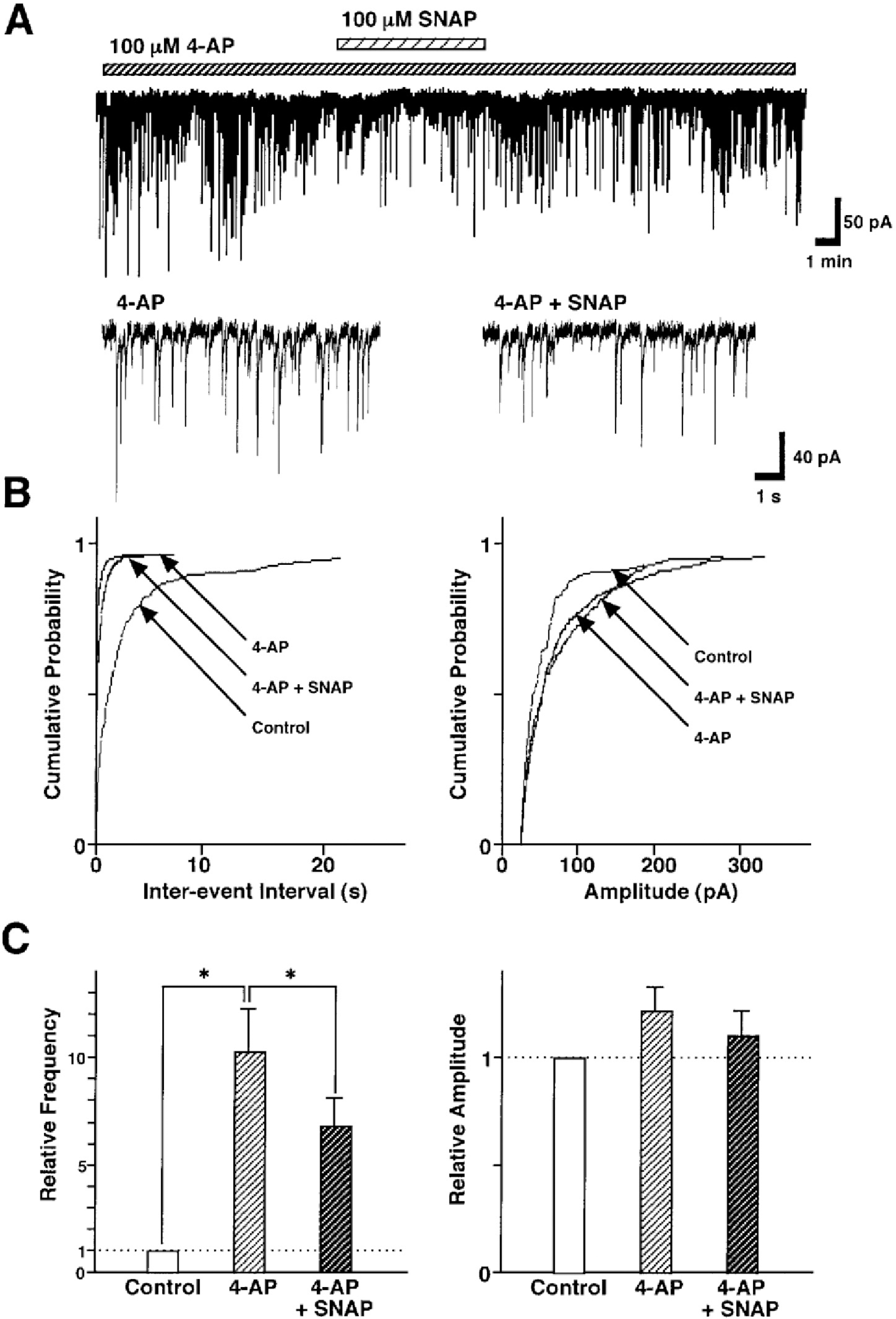
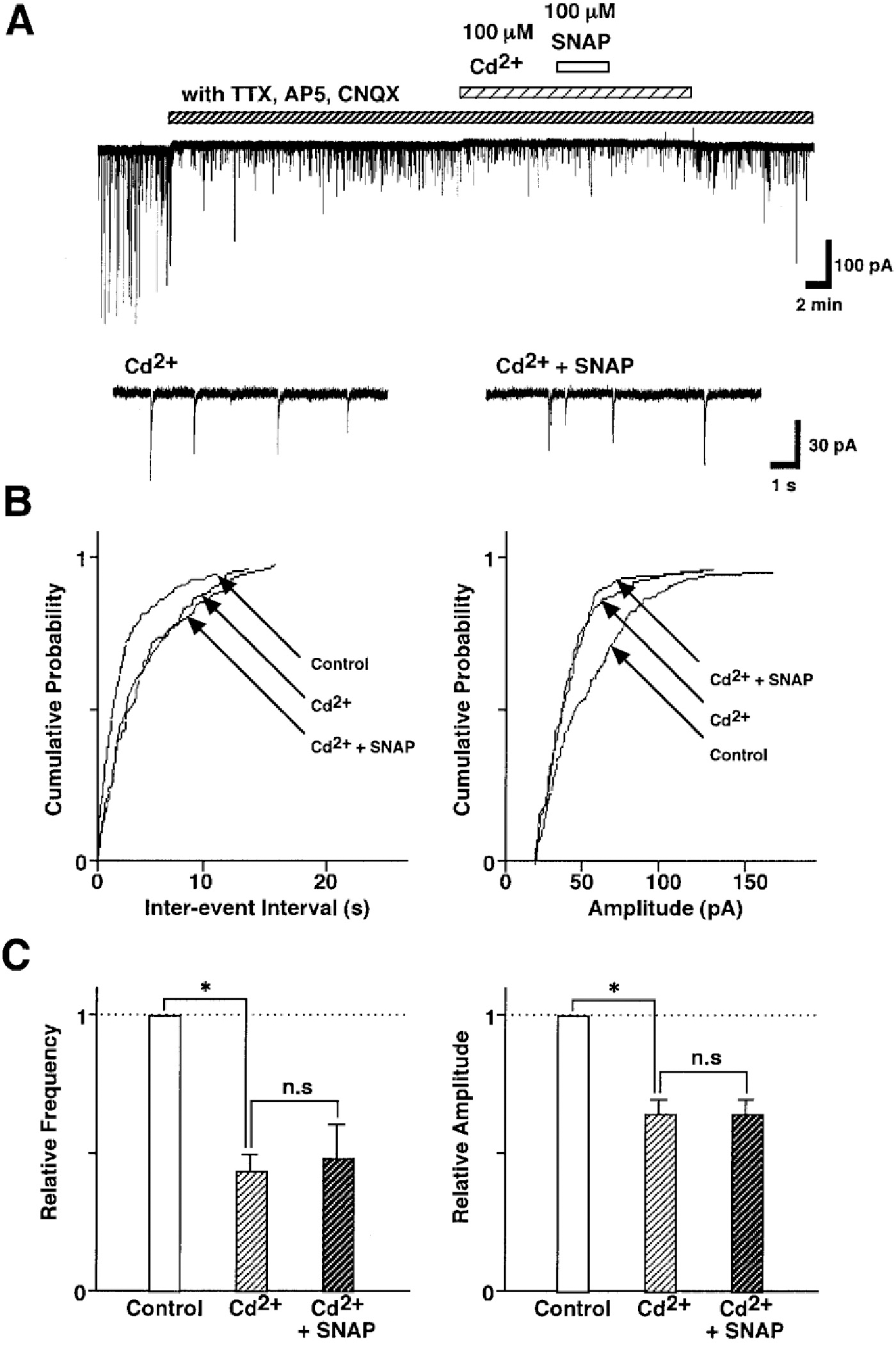
Fig. 6.
SNAP-induced inhibition of GABAergic sIPSCs is related to the presynaptic VDCCs. (A) Representative recording traces of sIPSCs shows the change in the SNAP effect in the external solution containing 100 μM Cd2+, a non-selective Ca2+ channel blocker. Insets represent typical traces with an expanded time scale. (B) Cumulative distributions for inter-event interval (left) and amplitude (right) of sIPSCs in the same neuron. p values indicate the results of K-S tests for frequency and amplitude. (C) All amplitudes and frequencies are normalized to the control sIPSCs. Each column is the mean of 6 neurons. Asterisks represent a statistically significant difference (∗p<0.05, paired two-tailed t-test). n.s indicates p>0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download