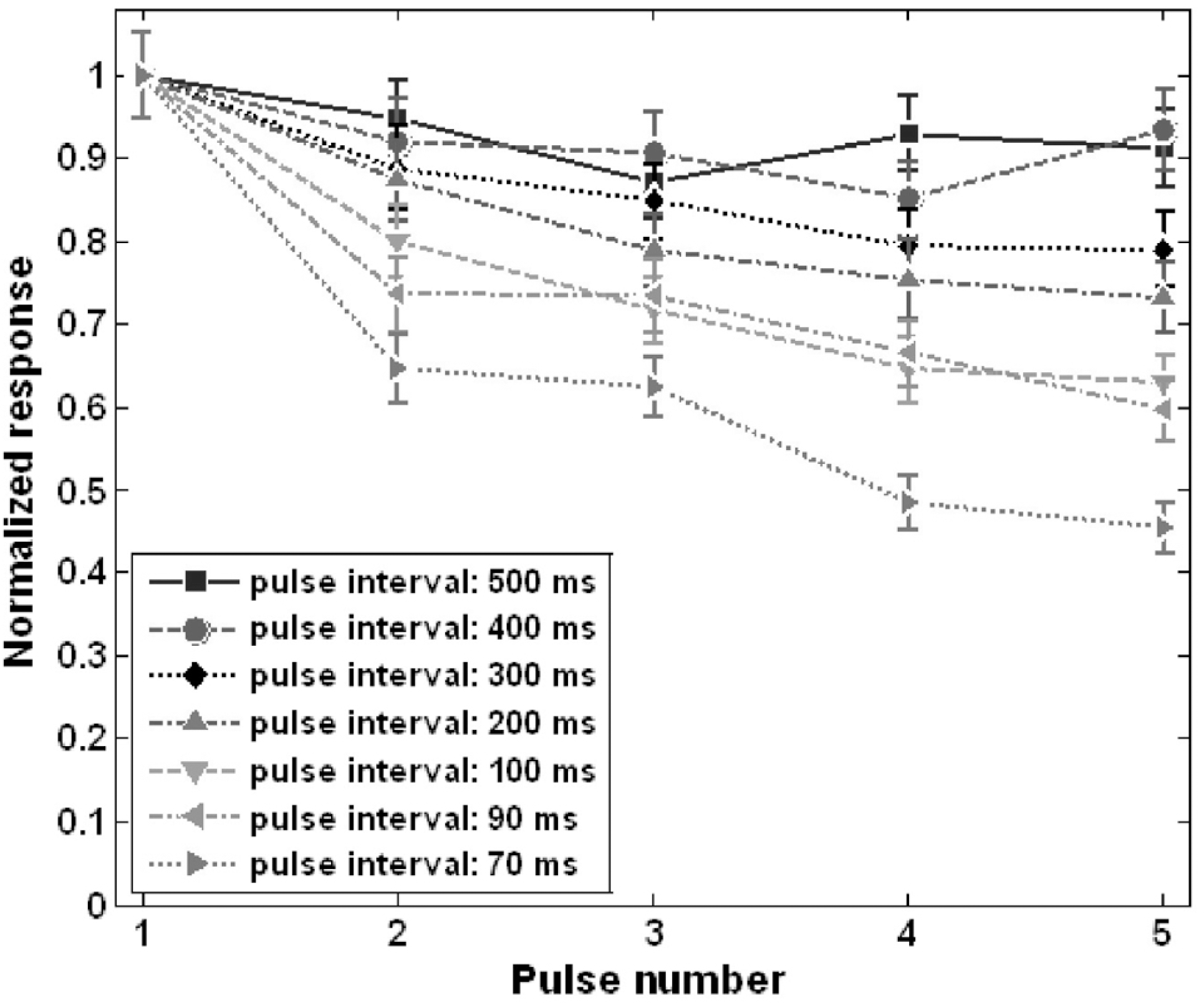
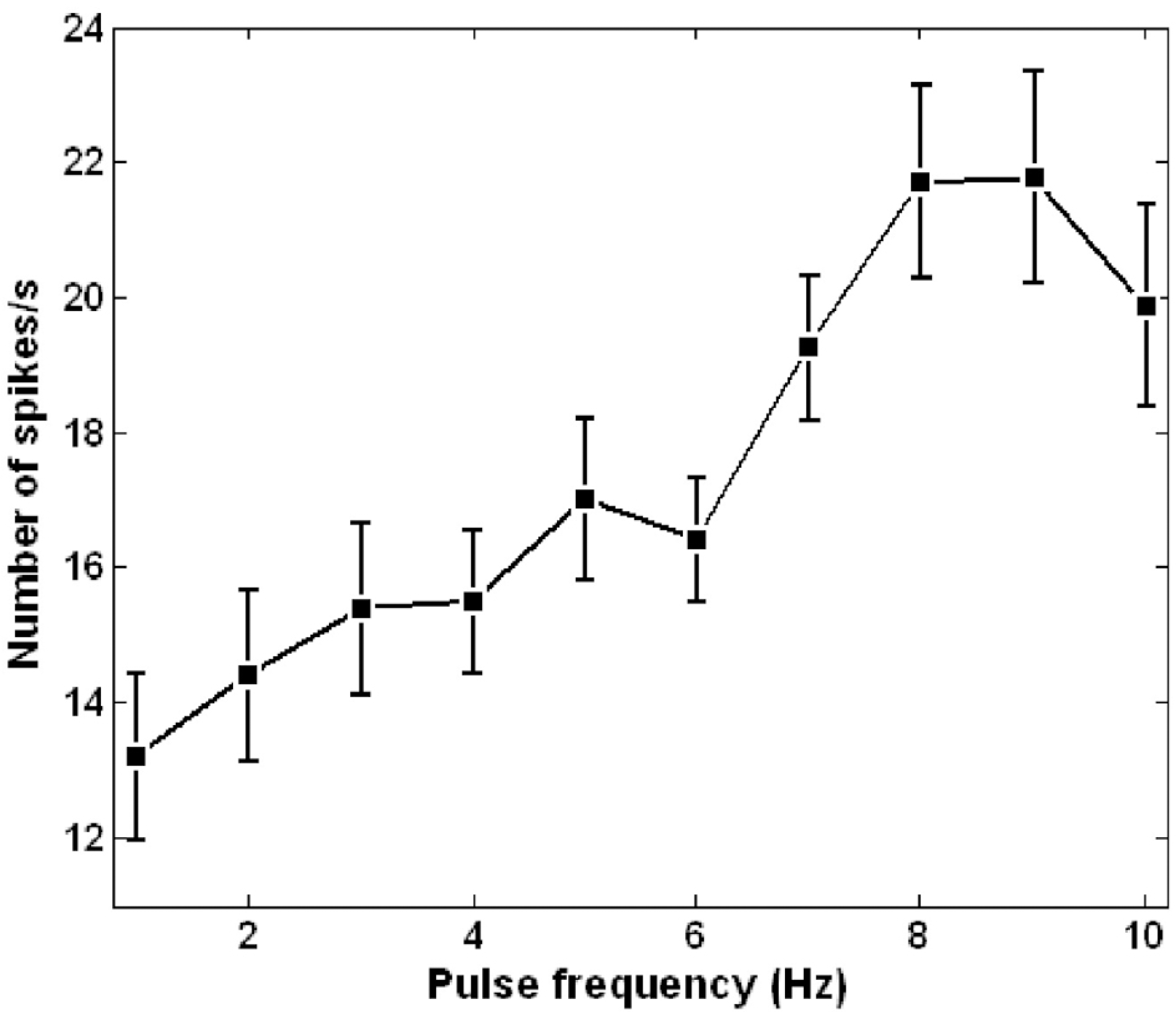
Abstract
For successful visual perception by visual prosthesis using electrical stimulation, it is essential to develop an effective stimulation strategy based on understanding of retinal ganglion cell (RGC) responses to electrical stimulation. We studied RGC responses to repetitive electrical stimulation pulses to develop a stimulation strategy using stimulation pulse frequency modulation. Retinal patches of photoreceptor-degenerated retinas from rd1 mice were attached to a planar multi-electrode array (MEA) and RGC spike trains responding to electrical stimulation pulse trains with various pulse frequencies were observed. RGC responses were strongly dependent on inter-pulse interval when it was varied from 500 to 10 ms. Although the evoked spikes were suppressed with increasing pulse rate, the number of evoked spikes were >60% of the maximal responses when the inter-pulse intervals exceeded 100 ms. Based on this, we investigated the modulation of evoked RGC firing rates while increasing the pulse frequency from 1 to 10 pulses per second (or Hz) to deduce the optimal pulse frequency range for modulation of RGC response strength. RGC response strength monotonically and linearly increased within the stimulation frequency of 1∼9 Hz. The results suggest that the evoked neural activities of RGCs in degenerated retina can be reliably controlled by pulse frequency modulation, and may be used as a stimulation strategy for visual neural prosthesis.
References
Ahuja AK., Behrend MR., Kuroda M., Humayun MS., Weiland JD. An in-vitro model of a retinal implant. IEEE Trans Biomed Eng. 55:1744–1753. 2008.
Fried SI., Hsueh HA., Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol. 95:970–978. 2006.

Hesse L., Schanze T., Wilms M., Eger M. Implantation of retina stimulation electrodes and recording of electrical stimulation responses in the visual cortex of the cat. Graef Arch Clin Exp Ophthal. 238:840–845. 2000.

Hornig R., Laube T., Walter P., Velikay-Parel M., Bornfeld N., Feucht M., Akguel H., Rössler G., Alteheld N., Notarp DL., Wyatt J., Richard G. A method and technical equipment for an acute human trial to evaluate retinal implant technology. J Neural Eng. 2:S129–S124. 2005.

Humayun MS., de Juan E Jr., Weiland JD., Dangnelie G., Katona S., Greenberg R., Suzuki S. Pattern electrical stimulation of the human retina. Vision Res. 39:2569–2576. 1999.

Humayun MS., Weiland JD., Fujii GY., Greenberg R., Williamson R., Little J., Mech B., Cimmarusti V., Boemel GV., Dagnelie G., de Juan E Jr. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 43:2573–2581. 2003.

Jensen RJ., Rizzo JF III. Responses of ganglion cells to repetitive electrical stimulation of the retina. J Neural Eng. 4:S1–S6. 2007.

Jensen RJ., Ziv OR., Rizzo JF. Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode. J Neural Eng. 2:S16–S21. 2005.

Kazemi M., Basham E., Sivaprakasam M., Wang G., Rodger D., Weiland J., Tai YC., Liu W., Humayun M. A test microchip for evaluation of hermetic packaging technology for biomedical prosthetic implants. Conf Proc IEEE Eng Med Biol Soc. 6:4093–4095. 2004.

Margolis DJ., Newkirk G., Euler T., Detwiler PB. Functional stability of retinal ganglion cells after degeneration-induced changes in synaptic input. J Neurosci. 28:6526–6536. 2008.

Merabet LB., Rizzo JF., Amedi A., Somers DC., Pascual-Leone A. What blindness can tell us about seeing again: merging neuroplasticity and neuroprosthesis. Nat Rev Neurosci. 6:71–77. 2005.
Rizzo JF III., Wyatt J., Loewenstein J., Kelly S., Shire D. Perceptual efficacy of electrical stimulation of human retina with a micro-electrode array during short-term surgical trials. Invest Ophth Vis Sci. 44:5362–5369. 2003.
Ryu SB., Lee JS., Ye JH., Goo YS., Kim CH., Kim KH. Analysis of neuronal activities of retinal ganglion cells of degenerated retinal evoked by electrical pulse stimulation. J Biomed Eng Res. 30:347–354. 2009a.
Ryu SB., Ye JH., Lee JS., Goo YS., Kim KH. Characterization of retinal ganglion cell activities evoked by temporally patterned electrical stimulation for the development of stimulus encoding strategies for retinal implants. Brain Res. 1275:33–42. 2009b.

Sekirnjak C., Hottowy P., Sher A., Dabrowski W., Litke AM., Chichilnisky EJ. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J Neurophysiol. 95:3311–3327. 2006.

Seo JM., Kim SJ., Chung H., Kim ET., Yu HG., Yu YS. Biocompatibility of polyimide microelectrode array for retinal stimulation. Mater Sci Eng C. 24:185–189. 2004.

Sivaprakasam M., Wentai L., Guoxing W., Weiland JD., Humayun MS. Architecture tradeoffs in high-density microstimulators for retinal prosthesis. IEEE Trans Circuits Syst I-Regul Pap. 52:2629–2641. 2005.

Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol. 99:1408–1421. 2008.

Stett A., Barth W., Weiss S., Haemmerle H., Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vis Res. 40:1785–1795. 2000.

Veraart C., Raftopoulos C., Mortmer JT., Delbeke J., Pins D., Michaus G., Vanlierde A., Parrini S., Wanet-Defalqu M. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res. 813:181–186. 1998.

Ye JH., Goo YS. The slow wave component of retinal activity in rd/rd mouse recorded with a multi-electrode array. Physiol Meas. 28:1079–1088. 2007.
Fig. 1.
(A) A typical raw waveform of spontaneous retinal activity. Rhythmic oscillatory behavior with ∼10 Hz frequency is clearly observable from the background. (B) A typical raw waveform recorded under stimulation. (C) Electrically-evoked RGC spike waveforms derived from the raw recording in (B) by highpass filtering (second order Butterworth filter, cutoff frequency: 100 Hz).
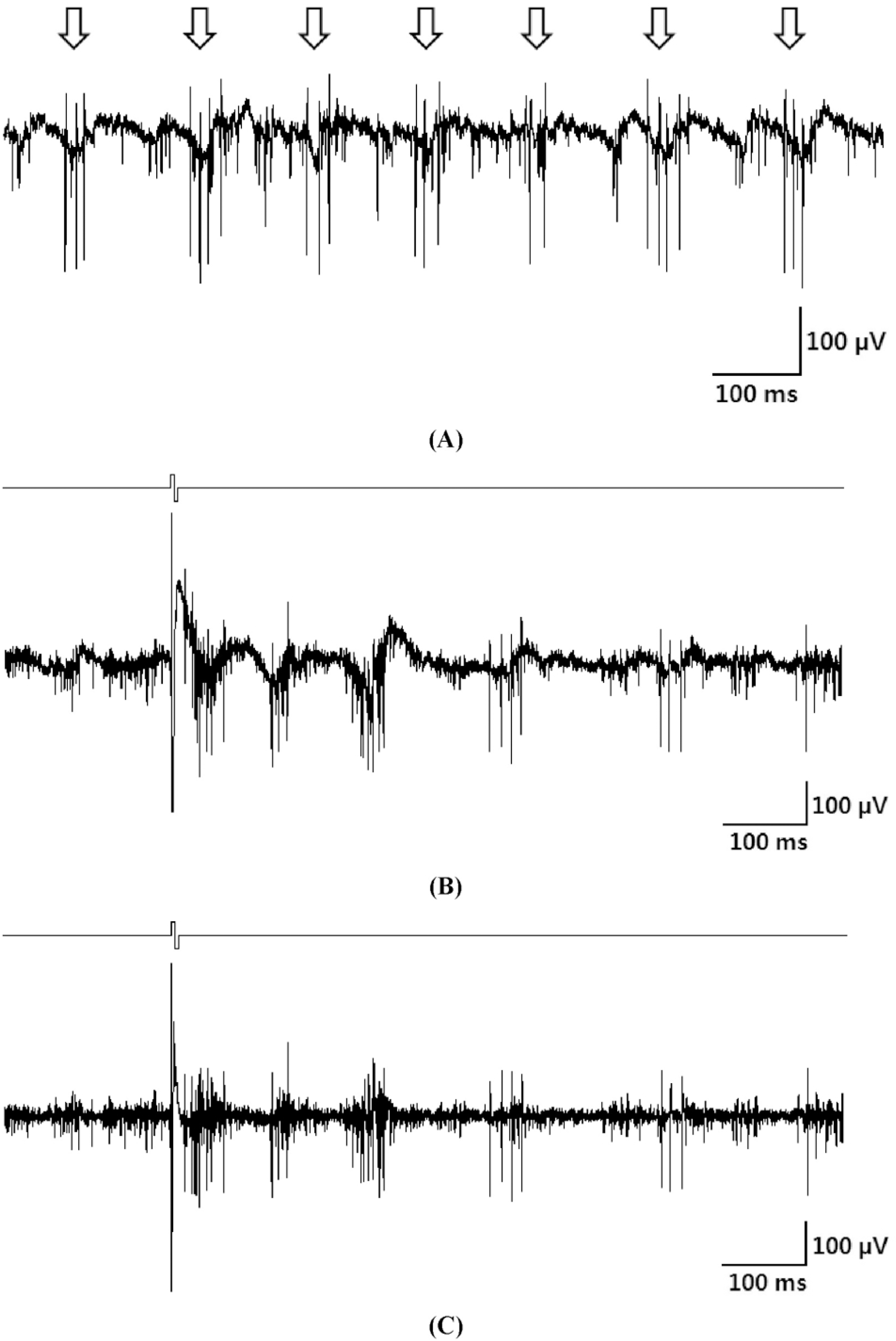
Fig. 2.
Short-latency RGC spikes. (A) Inter-pulse interval: 500 ms, (B) inter-pulse interval: 10 ms (Thin solid line: before TTX treatment. Thick dotted line: after TTX treatment. Template subtraction result: indicated by arrows.).
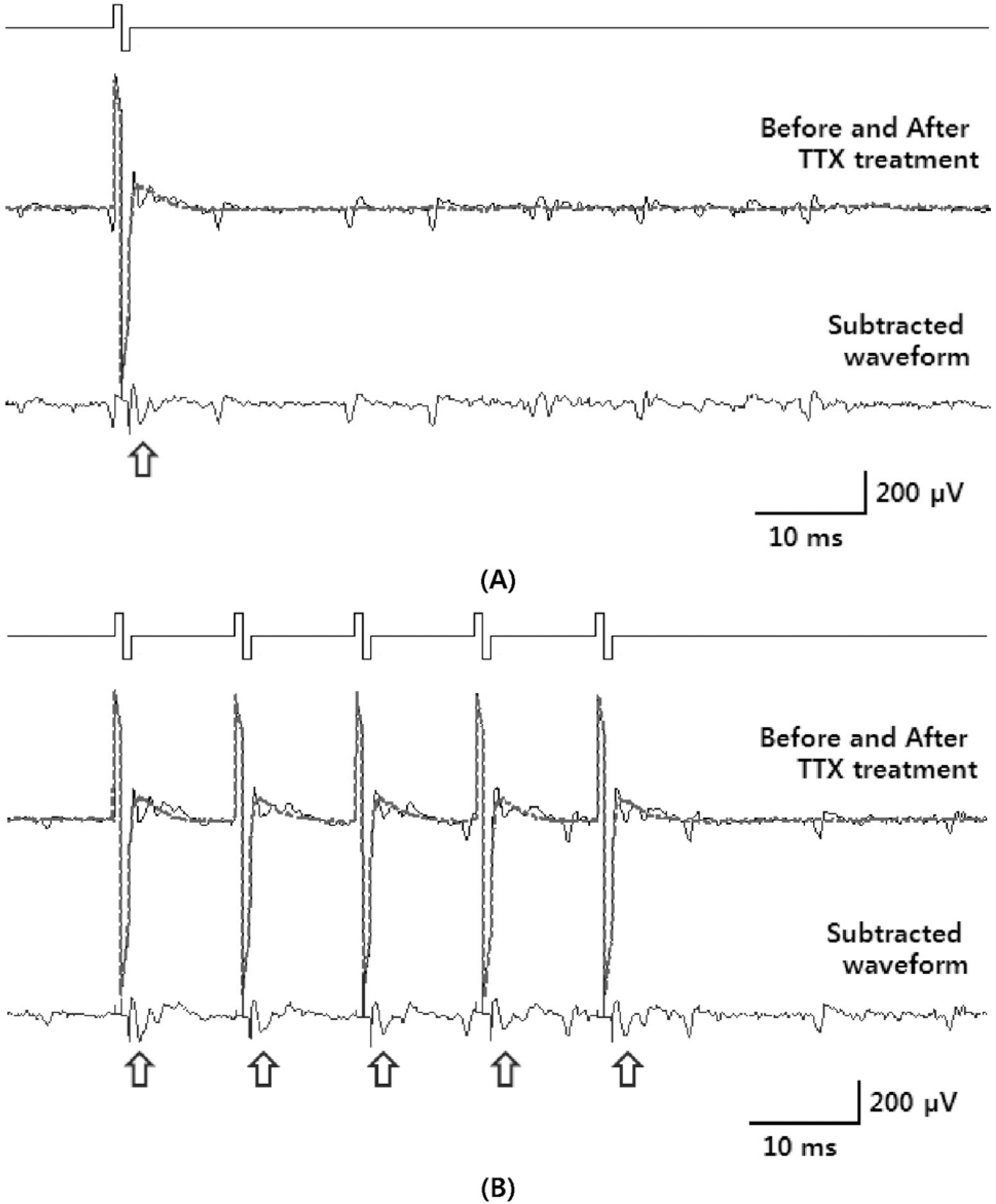
Fig. 3.
Electrically-evoked RGC responses when a pair of pulses was applied. (A) Inter-pulse interval: 500 ms. (B) Inter-pulse interval: 100 ms. (C) Inter-pulse interval: 50 ms. (D) Inter-pulse interval: 10 ms.
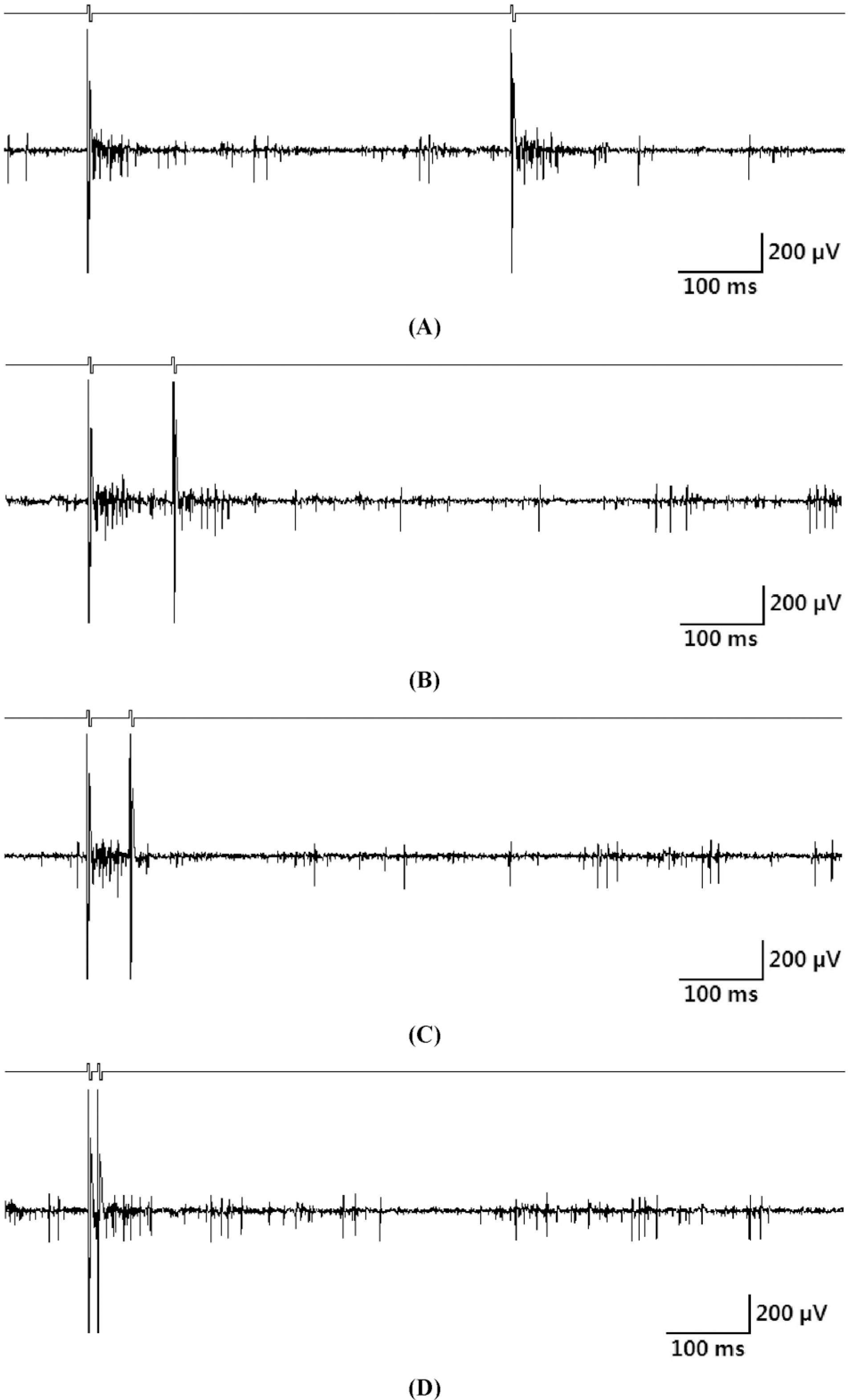
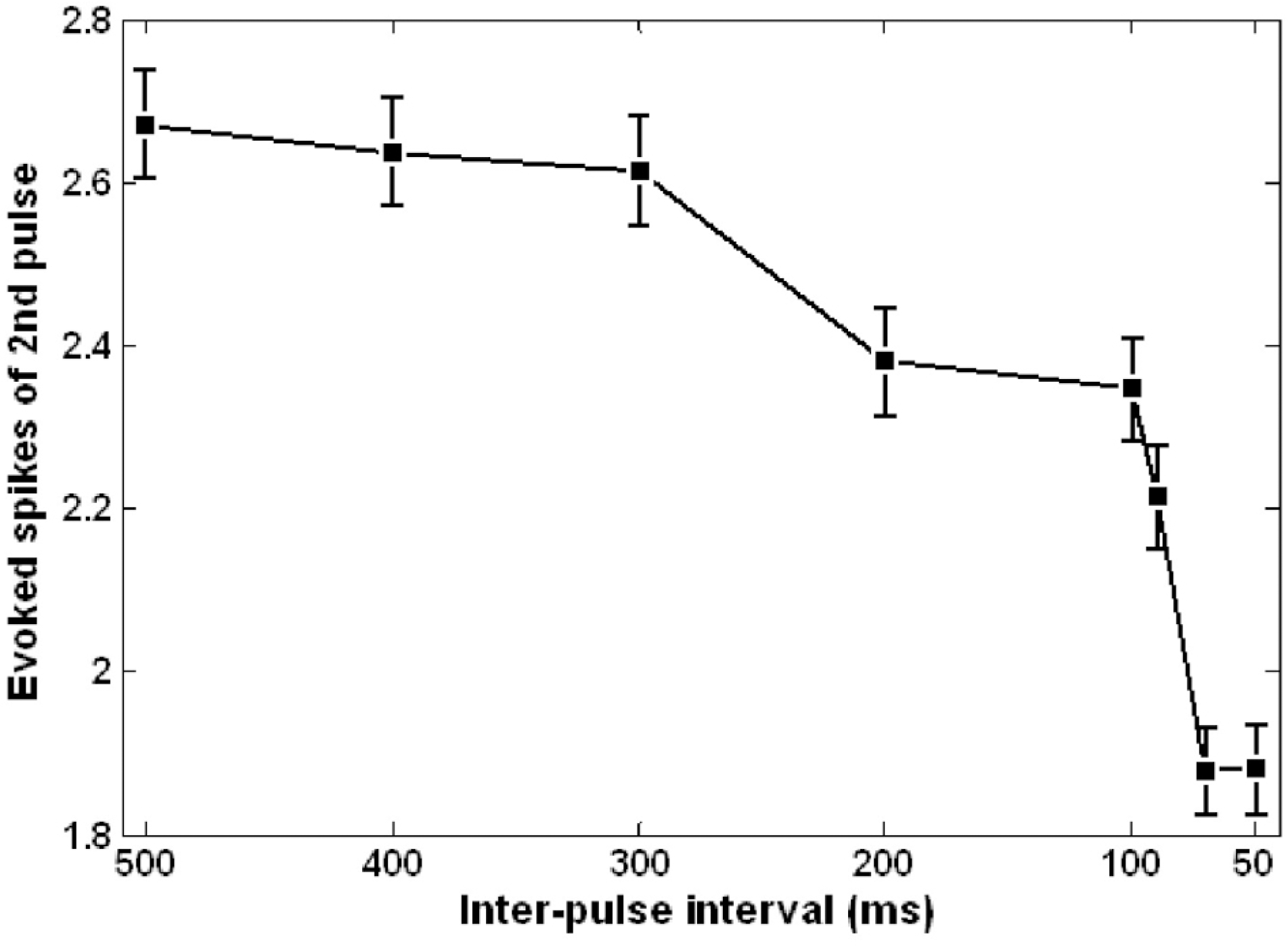
Fig. 4.
The number of RGC spikes evoked by second pulses in the pulse pairs plotted as a function of inter-pulse interval (500∼50 ms). Each data point was obtained from the average of 75 RGCs. Error bar denotes standard error.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download