Abstract
The role of apurinic/apyrimidinic endonuclease1/redox factor-1 (Ref-1) on the lead (Pb)-induced cellular response was investigated in the cultured endothelial cells. Pb caused progressive cellular death in endothelial cells, which occurred in a concentration- and time-dependent manner. However, Ref-1 overexpression with AdRef-1 significantly inhibited Pb-induced cell death in the endothelial cells. Also the overexpression of Ref-1 significantly suppressed Pb-induced superoxide and hydrogen peroxide elevation in the endothelial cells. Pb exposure induced the downregulation of catalase, it was inhibited by the Ref-1 overexpression in the endothelial cells. Taken together, our data suggests that the overexpression of Ref-1 inhibited Pb-induced cell death via the upregulation of catalase in the cultured endothelial cells.
Go to : 
References
Adhikari N., Sinha N., Narayan R., Saxena DK. Lead-induced cell death in testes of young rats. J Appl Toxicol. 21:275–277. 2001.

Angkeow P., Deshpande SS., Qi B., Liu YX., Park YC., Jeon BH., Ozaki M., Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 9:717–725. 2002.

Cheng YJ., Yang BC., Hsieh WC., Huang BM., Liu MY. Enhancement of TNF-alpha expression does not trigger apoptosis upon exposure of glial cells to lead and lipopolysaccharide. Toxicology. 178:183–191. 2002.
Chetty CS., Vemuri MC., Reddy GR., Suresh C. Protective effect of 17-beta-estradiol in human neurocellular models of lead exposure. Neurotoxicology. 28:396–401. 2007.
Chovolou Y., Watjen W., Kampkotter A., Kahl R. Resistance to tumor necrosis factor-alpha (TNF-alpha)-induced apoptosis in rat hepatoma cells expressing TNF-alpha is linked to low antioxidant enzyme expression. J Biol Chem. 278:29626–29632. 2003.
Clerch LB., Massaro D. Oxidation-reduction-sensitive binding of lung protein to rat catalase mRNA. J Biol Chem. 267:2853–2855. 1992.

Clerch LB., Wright A., Chung DJ., Massaro D. Early divergent lung antioxidant enzyme expression in response to lipopolysaccharide. Am J Physiol. 271:L949–954. 1996.

Demple B., Herman T., Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 88:11450–11454. 1991.

Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 1:529–539. 2001.
Fischer JL., Lancia JK., Mathur A., Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 26:899–904. 2006.
Fox DA., He L., Poblenz AT., Medrano CJ., Blocker YS., Srivastava D. Lead-induced alterations in retinal cGMP phosphodiesterase trigger calcium overload, mitochondrial dysfunction and rod photoreceptor apoptosis. Toxicol Lett. 102–103:359–361. 1998.

Gonick HC., Ding Y., Bondy SC., Ni Z., Vaziri ND. Lead-induced hypertension: interplay of nitric oxide and reactive oxygen species. Hypertension. 30:1487–1492. 1997.
Grosch S., Fritz G., Kaina B. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 58:4410–4416. 1998.
Hunaiti A., Soud M., Khalil A. Lead concentration and the level of glutathione, glutathione S-transferase, reductase and peroxidase in the blood of some occupational workers from Irbid City, Jordan. Sci Total Environ. 170:95–100. 1995.

Jeon BH., Gupta G., Park YC., Qi B., Haile A., Khanday FA., Liu YX., Kim JM., Ozaki M., White AR., Berkowitz DE., Irani K. Apurinic/apyrmidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 95:902–910. 2004.

Kim CS., Son SJ., Kim EK., Kim SN., Yoo DG., Kim HS., Ryoo SW., Lee SD., Irani K., Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 69:520–526. 2006.

Lee HM., Jeon BH., Won KJ., Lee CK., Park TK., Choi WS., Bae YM., Kim HS., Lee SK., Park SH., Irani K., Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 104:219–227. 2009.
Lupertz R., Chovolou Y., Kampkotter A., Watjen W., Kahl R. Catalase overexpression impairs TNF-alpha induced NF-kappaB activation and sensitizes MCF-7 cells against TNF-alpha. J Cell Biochem. 103:1497–1511. 2008.
Massanyi P., Lukac N., Makarevich AV., Chrenek P., Forgacs Z., Zakrzewski M., Stawarz R., Toman R., Lazor P., Flesarova S. Lead-induced alterations in rat kidneys and testes in vivo. J Environ Sci Health A Tox Hazard Subst Environ Eng. 42:671–676. 2007.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. 1983.

Nakamura H., Nakamura K., Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 15:351–369. 1997.

Nenoi M., Ichimura S., Mita K., Yukawa O., Cartwright IL. Regulation of the catalase gene promoter by Sp1, CCAAT-recognizing factors, and a WT1/Egr-related factor in hydrogen peroxide-resistant HP100 cells. Cancer Res. 61:5885–5894. 2001.
Ni Z., Hou S., Barton CH., Vaziri ND. Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney Int. 66:2329–2336. 2004.

Ozaki M., Suzuki S., Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 16:889–890. 2002.
Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 11:114–127. 2006.
Sharp DS., Osterloh J., Becker CE., Bernard B., Smith AH., Fisher JM., Syme SL., Holman BL., Johnston T. Blood pressure and blood lead concentration in bus drivers. Environ Health Perspect. 78:131–137. 1988.

Silber JR., Bobola MS., Blank A., Schoeler KD., Haroldson PD., Huynh MB., Kolstoe DD. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res. 8:3008–3018. 2002.
Song YJ., Lee JY., Joo HK., Kim HS., Lee SK., Lee KH., Cho CH., Park JB., Jeon BH. Tat-APE1/ref-1 protein inhibits TNF-alpha-induced endothelial cell activation. Biochem Biophys Res Commun. 368:68–73. 2008.
Stacchiotti A., Morandini F., Bettoni F., Schena I., Lavazza A., Grigolato PG., Apostoli P., Rezzani R., Aleo MF. Stress proteins and oxidative damage in a renal derived cell line exposed to inorganic mercury and lead. Toxicology. 264:215–224. 2009.

Suresh C., Dennis AO., Heinz J., Vemuri MC., Chetty CS. Melatonin protection against lead-induced changes in human neuroblastoma cell cultures. Int J Toxicol. 25:459–464. 2006.

Tell G., Damante G., Caldwell D., Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 7:367–384. 2005.

Tomicic M., Eschbach E., Kaina B. Expression of yeast but not human apurinic/apyrimidinic endonuclease renders Chinese hamster cells more resistant to DNA damaging agents. Mutat Res. 383:155–165. 1997.

Ushakova T., Melkonyan H., Nikonova L., Afanasyev V., Gaziev AI., Mudrik N., Bradbury R., Gogvadze V. Modification of gene expression by dietary antioxidants in radiation-induced apoptosis of mice splenocytes. Free Radic Biol Med. 26:887–891. 1999.

Vaziri ND. Pathogenesis of lead-induced hypertension: role of oxidative stress. J Hypertens Suppl. 20:S15–20. 2002.
Vaziri ND., Lin CY., Farmand F., Sindhu RK. Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int. 63:186–194. 2003.

Wang L., Wang H., Hu M., Cao J., Chen D., Liu Z. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 83:417–427. 2009.

Go to : 
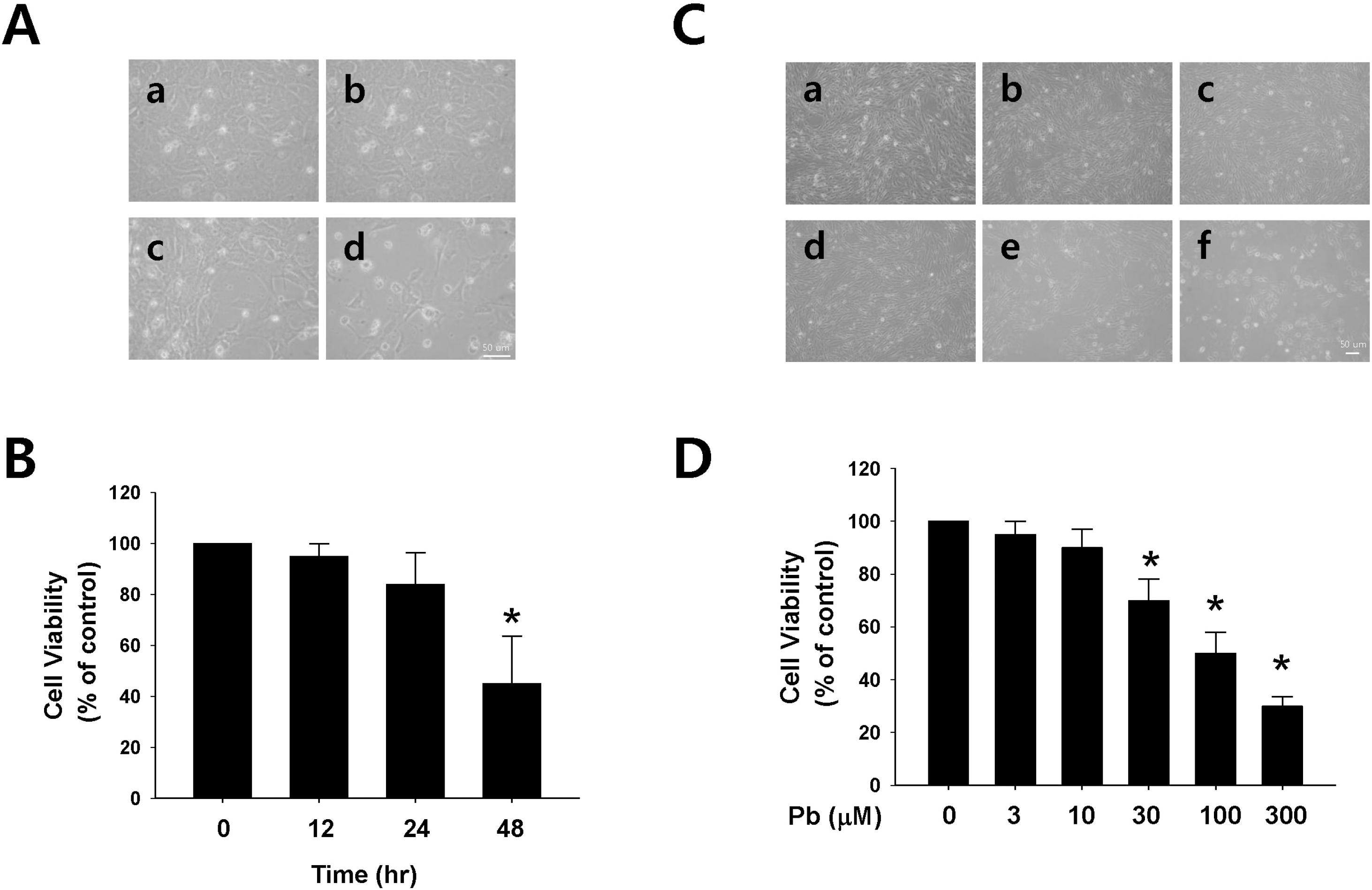 | Fig. 1.Effect of lead (Pb) on endothelial cell viability. (A) Typical endothelial cell morphology after treatment with Pb acetate (30 μM) for 12 ∼48 hr (a, control; b, 12 hr; c, 24 hr; d, 48 hr). (B) Summarized data are plotted in Fig. 1B. (C) Typical endothelial cell morphology after treatment with Pb acetate for 24 hr. (a, control; b, 3 μM of Pb; c, 10 μM of Pb; d, 30 μM of Pb; e, 100 μM of Pb; f, 300 μM of Pb). (D) Summarized data was plotted in Fig. 1D. Total acetate concentration (300 μM) was balanced with Na acetate in Fig. 1C. Endothelial cell viability was measured via a MTT assay as described in the Materials and Methods section. The data are expressed as the means± SEM for 3 separate experiments. ∗p <0.05 compared with control. |
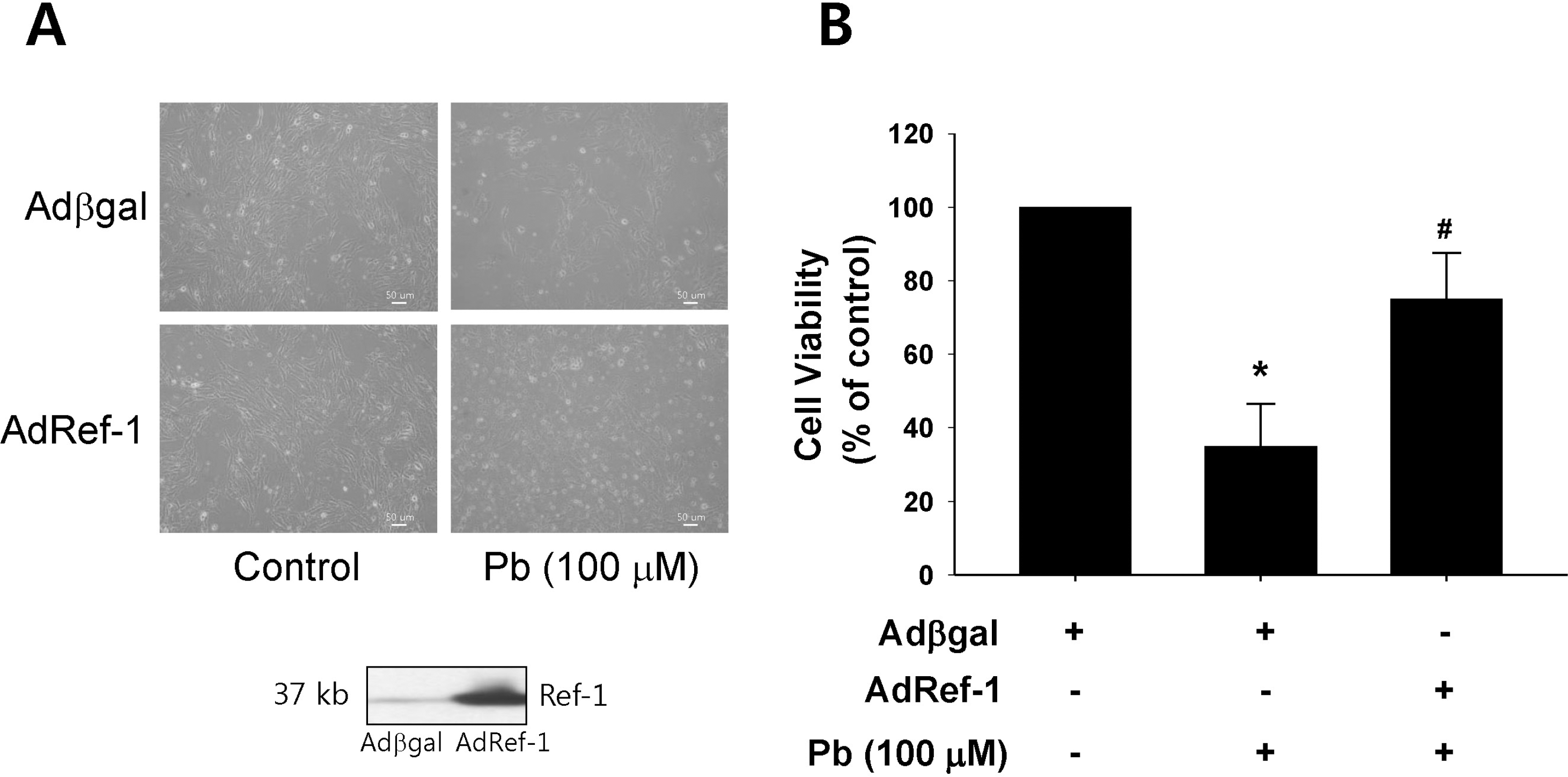 | Fig. 2.Ref-1 overexpression inhibited lead (Pb) induced endothelial cell death. (A) Endothelial cell morphology in the Pb (100 μM)-treated endothelial cells which were transfected with Adβgal or AdRef-1. Adenoviral transfection of AdRef-1 successfully overexpressed Ref-1 in the endothelial cells as compared with Adβgal. (B) Ref-1 overexpression induced by AdRef-1 inhibited Pb (100 μM)-induced endothelial cell death (in the bottom of Fig. 2A). (B) Summarized data was plotted in Fig. 2B. Endothelial cell viability was measured with an MTT assay as described in the Materials and Methods section. The data are expressed as the means± SEM for 4 separate experiments. ∗p < 0.05 compared with control cells. #p<0.05 compared with Adβgal+Pb. |
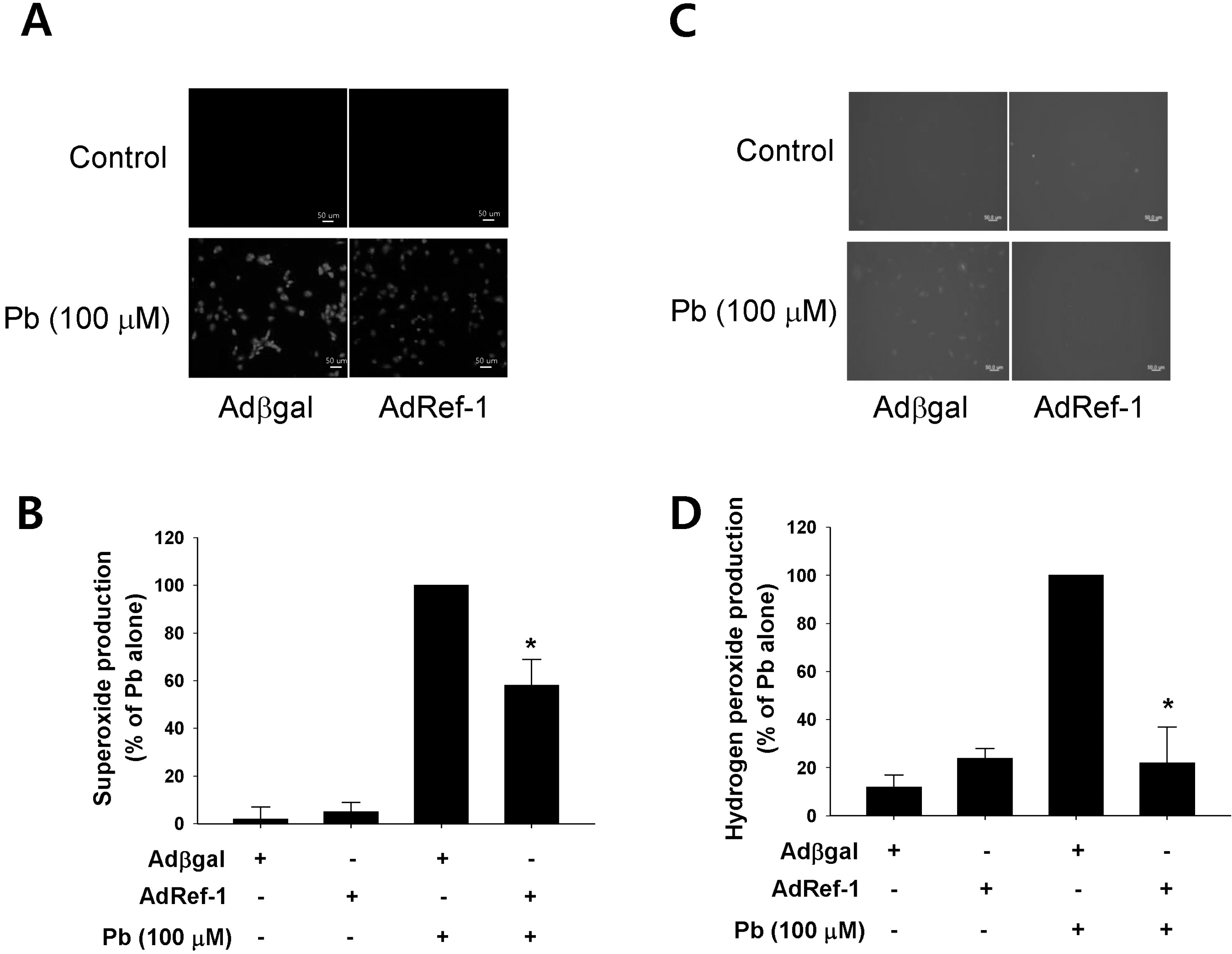 | Fig. 3.Ref-1 overexpression inhibited lead (Pb)-induced superoxide and hydrogen peroxide production. Pb (100 μM) was administered for 24 hr in the endothelial cells transfected with Adβgal or AdRef-1. Intracellular superoxide (A) and hydrogen peroxide production (C) were evaluated using the superoxide-sensitive fluorophore dihydroethidine (DHE) and the peroxide-sensitive fluorophore 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA). (B, D) The summarized data was plotted in Figs. 3B and 3D. The data are expressed as the means± SEM for 3 separate experiments. ∗p <0.05 compared with Adβgal + Pb. |
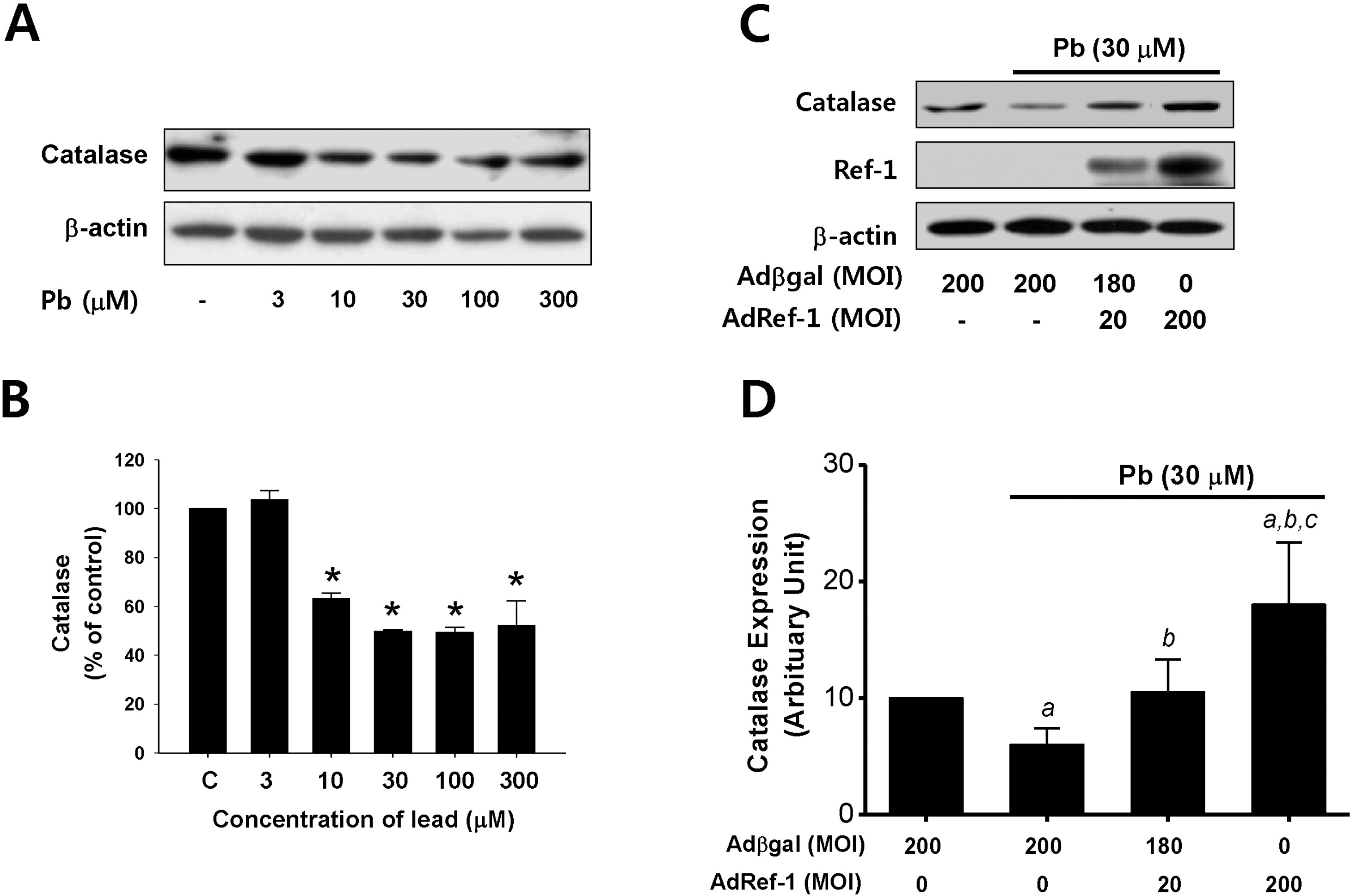 | Fig. 4.Ref-1 inhibited lead-induced catalase suppression in the endothelial cells. (A) Lead (Pb) treatment resulted in the downregulation of catalase expression. Endothelial cells were exposed to lead (3∼300 μM) for 24 hr. Western blot analysis for catalase expression was conducted. β-actin was used as a loading control. (B) Summarized data was plotted in Fig. 4B. ap<0.05 compared with control. The data were expressed as the means±SEM for 3 separate experiments. (C) Effect of Ref-1 on the Pb-induced suppression of catalase expression in the endothelial cells. Endothelial cells were exposed by lead (30 μM) for 24 hr in the endothelial cells transfected with 20 or 200 MOI of AdRef-1. A total of 200 MOI of adenoviral transfection was balanced with Adβgal. (D) The summarized data was plotted in Fig. 4D. The data were expressed as the means± SEM for 3 separate experiments. ap <0.05 compared with Adβgal 200 MOI, bp<0.05 compared with AdβGal + Pb, and cp<0.05 compared with AdRef-1 20 MOI + Pb. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download