Abstract
Acute pancreatitis is a multifactorial disease associated with the premature activation of digestive enzymes. The genes expressed in pancreatic acinar cells determine the severity of the disease. The present study determined the differentially expressed genes in pancreatic acinar cells treated with cerulein as an in vitro model of acute pancreatitis. Pancreatic acinar AR42J cells were stimulated with 10–8 M cerulein for 4 h, and genes with altered expression were identified using a cDNA microarray for 4,000 rat genes and validated by real-time PCR. These genes showed a 2.5-fold or higher increase with cerulein: lithostatin, guanylate cyclase, myosin light chain kinase 2, cathepsin C, progestin-induced protein, and pancreatic trypsin 2. Stathin 1 and ribosomal protein S13 showed a 2.5-fold or higher decreases in expression. Real-time PCR analysis showed time-dependent alterations of these genes. Using commercially available antibodies specific for guanylate cyclase, myosin light chain kinase 2, and cathepsin C, a time-dependent increase in these proteins were observed by Western blotting. Thus, disturbances in proliferation, differentiation, cytoskeleton arrangement, enzyme activity, and secretion may be underlying mechanisms of acute pancreatitis.
Go to : 
References
Barone S., Okaya T., Rudich S., Petrovic S., Tenrani K., Wang Z., Zahedi K., Casero RA., Lentsch AB., Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 289:C826–C835. 2005.

Belmont LD., Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 84:623–631. 1996.

Biéche I., Maucuer A., Laurendeau I., Lachkar S., Spano AJ., Frankfurter A., Lévy P., Manceau V., Sobel A., Vidaud M., Curmi PA. Expression of stathmin family genes in human tissues: non-neural-restricted expression for SCLIP. Genomics. 81:400–410. 2003.

Bissonnette M., Kuhn D., de Lanerolle P. Purification and characterization of myosin light-chain kinase from the rat pancreas. Biochem J. 258:739–747. 1989.

Bulun SE., Cheng YH., Yin P., Imir G., Utsunomiya H., Attar E., Innes J., Julie Kim J. Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol Cell Endocrinol. 248:94–103. 2006.

Carnevale RP., Proietti CJ., Salatino M., Urtreger A., Peluffo G., Edwards DP., Boonyaratanakornkit V., Charreau EH., Bal de Kier Joffe E., Schillaci R., Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 21:1335–1358. 2007.
Chen N., Zou J., Wang S., Ye Y., Huang Y., Gadda G., Yang JJ. Designing protease sensors for real-time imaging of trypsin activation in pancreatic acinar cells. Biochemistry. 48:3519–3526. 2009.
Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci USA. 91:11075–11079. 1994.

Choi JY., Kim KH. Effects of small molecular antioxidants on cerulein-induced acute pancreatitis in rat. Korean J Physiol Pharmacol. 2:629–635. 1998.
Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 62:156–159. 1987.

Cukras AR., Green R. Multiple effects of S13 in modulating the strength of intersubunit interactions in the ribosome during translation. J Mol Biol. 349:47–59. 2005.

Drewett JG., Garbers DL. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 15:135–162. 1994.

Freshney RI. Culture of Animal Cells; A Manual for Basic Technique. 3th ed.John Wiley and Sons Inc;New York: p. p. 71–103. 1994.
Giancotti FG., Mainiero F. Integrin-mediated adhesion and signaling in tumorigenesis. Biochim Biopys Acta. 1198:47–64. 1994.

Gorelick FS., Otani T. Mechanisms of intracellular zymogen activation. Baillieres Best Pract Res Clin Gastroenterol. 13:227–240. 1999.

Grady T., Dabroski A., Williams JA., Logsdon CD. Stress-activated protein kinase activation is the earliest direct correlate to the induction of secretagogue-induced pancreatitis in rats. Biochem Biophys Res Commun. 227:1–7. 1996.

Graf R., Schiesser M., Scheele GA., Marquardt K., Frick TW., Mumann RW., Bimmler D. A family of 16-kDa pancreatic secretory stress proteins form highly organized fibrillar structures upon tryptic activation. J Biol Chem. 276:21028–21038. 2001.

Hartupee JC., Zhang H., Bonaldo MF., Soares MB., Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta. 1518:287–293. 2001.

Iancu C., Mistry SJ., Arkin S., Wallenstein S., Atweh GF. Effects of stathmin inhibition on the mitotic spindle. J Cell Sci. 114:909–916. 2001.

Jensen RT., Wank SA., Rowley WH., Sato S., Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 10:418–423. 1989.

Ji B., Chen X-Q., Misek DE., Kuick R., Hanash S., Ernst S., Najarian R., Logsdin CD. Pancreatic gene expression during the initiation of acute pancreatitis: identification of EGR-1 as a key regulator. Physiol Genomics. 14:59–72. 2003.

Ju KD., Yu JH., Kim H., Kim KH. Role of mitogen-activated protein kinases, NF-κB, and AP-1 on cerulein-induced IL-8 expression in pancreatic acinar cells. Ann N Y Acad Sci. 1090:368–374. 2006.

Kim H., Kim KH. Secretory response of cultured acinar cells of rat pancreas to cholecystokinin. Yonsei Medical J. 37:405–411. 1996.

Kim JY., Kim KH., Lee JA., Namkung W., Sun AQ., Anathanarayanan M., Suchy FJ., Shin DM., Muallem S., Lee MG. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology. 122:1941–1953. 2002.
Kim H., Seo JY., Cho SH., Kim KH. Lipid peroxidation, NF-κB activation and cytokine production in neutrophil-stimulated pancreatic acinar cells. Kor J Physiol Pharmacol. 3:521–528. 1999.
Koivunen E., Saksela O., Itkonen O., Osman S., Huhtala ML., Stenman UH. Human colon carcinoma, fibrosarcoma and leukemia cell lines produce tumor-associated trypsinogen. Int J Cancer. 47:592–596. 1991.

Koppel J., Loyer P., Maucuer A., Rehak P., Manceau V., Guguen-Guillouzo C., Sobel A. Induction of stathmin expression during liver regeneration. FEBS Lett. 331:65–70. 1993.

Kurita T., Beitel LK., Cooke PS., Lydon GR., Cunha JP. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 62:831–838. 2000.
Larsson N., Melander H., Marklund U., Osterman O., Gullberg M. G2/M transition requires multisite phosphorylation of oncoprotein 18 by two distinct protein kinase systems. J Biol Chem. 270:14175–14183. 1995.

Lee JW., Kim KH., Kim H. Role of vascular endothelial growth factor-D (VEGF-D) on IL-6 expression in cerulein-stimulated pancreatic acinar cells. Ann NY Acad Sci. 1095:129–133. 2007.

Lee JW., Seo J., Kim H., Chung JB., Kim KH. Signal transduction of cerulein-induced cytokine expression and apoptosis in pancreatic acinar cells. Ann NY Acad Sci. 1010:104–108. 2003.

Lerch MM., Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 15:159–170. 1994.
Lowe DG. Human natriuretic peptide receptor-A guanylyl cyclase is self-associated prior to hormone binding. Biochemistry. 31:10421–10425. 1992.

Melhem RF., Strahler JR., Hailat N., Zhu XX., Hanash SM. Involvement of OP18 in cell proliferation. Biochem Biophys Res Commun. 179:1649–1655. 1991.

Multigner L., De Caro A., Lombardo D., Campese D., Saries H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochem Biophys Res Commun. 110:69–74. 1983.

Namkung W., Han W., Luo X., Muallem S., Cho KH., Kim KH., Lee MG. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology. 126:1844–1859. 2004.

Nieuwenhuizen AG., Schuiling GA., Liem SM., Moes H., Koiter TR., Uilenbroek JT. Progesterone stimulates pancreatic cell proliferation in vivo. Eur J Endocrinol. 140:256–263. 1999.

Noller HF., Hoang L., Fredrick K. The 30S ribosomal P site: a function of 16S rRNA. FEBS Lett. 579:855–858. 2005.

Patard L., Lallemand JY., Stoven V. An insight into the role of human pancreatic lithostathine. JOP. 4:92–103. 2003.
Piotrowski Z., Mysliwiec P., Gryko M., Ostrowska H., Baltaziak M. Chymotrypsin-like activity in rat tissues in experimental acute pancreatitis. Rocz Akad Med Bialymst. 48:61–65. 2003.
Poole AJ., Li Y., Kim Y., Lin SC., Lee WH., Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 314:1467–1470. 2006.
Potter LR., Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 267:14531–14534. 1992.

Rider V., Isuzugawa K., Twarog M., Jones S., Cameron B., Imakawa K., Fang J. Progesterone initiates Wnt-beta-catenin signaling but estradiol is required for nuclear activation and synchronous proliferation of rat uterine stromal cells. J Endocrinol. 191:537–548. 2006.
Rojas-Espinosa O., Arce-Paredez P., Dannenberg AM., Kamaenetz RL. Macrophage esterase: identification, purification and properties of a chymotrypsin-like esterase from lung that hydrolyses and transfers nonpolar amino acid esters. Biochim Biophys Acta. 22:161–179. 1975.

Rubin CI., Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 93:242–250. 2004.

Rubin CI., French DL., Atweh GF. Stathmin expression and megakaryocyte differentiation: a potential role in polyploidy. Exp Hematol. 31:389–397. 2003.

Sato S., Stark HA., Martinez J., Beaven M A., Jensen R T., Gardner JD. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 257(Gastrointest Liver Physiol 20):G202–G209. 1989.

Schoenberg MH., Bruchler M., Gaspar M., Stinner A., Younes M., Melzner I., Bültmann B., Beger HG. Oxygen free radicals in acute pancreatitis of the rat. Gut. 31:1138–1143. 1990.

Seidler NW., Jona I., Vegh M., Martonos A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 264:17816–17823. 1989.
Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 13:213–225. 1999.

Sternfeld L., Krause E., Guse AH., Schulz I. Hormonal control of ADP-ribosyl cyclase activity in pancreatic acinar cells from rats. J Biol Chem. 278:33629–33636. 2003.

Terazono K., Yamamoto H., Takasawa S., Shiga S., Yonemura Y., Tochino Y. A novel gene activated in regenerating islets. J Biol Chem. 263:2111–2114. 1988.

Violette S., Festor E., Pandreu-Vasile I., Mitchell V., Adida C., Lesuffleur T. Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer. 103:185–193. 2003.

Virlos I., Mazzon E., Serraino I., Genovese T., Di Paola R., Thiemerman C., Siriwardena A., Cuzzocrea S. Calpain I inhibitor ameliorates the indices of disease severity in a murine model of cerulein-induced acute pancreatitis. Intensive Care Med. 30:1645–1651. 2004.

Walz DA., Fenton JW. The role of thrombin in tumor cell metastasis. Invasion Metastasis. 14:303–308. 1994.
Watanabe T., Yonekura H., Terazono K., Yamamoto H., Okamoto H. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. The reg protein, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. J Biol Chem. 265:7432–7439. 1990.

Wedel BJ., Garbers DL. New insights on the functions of the guanylyl cyclase receptors. FEBS Lett. 410:29–33. 1997.

Yang F., Liu WP., He MX., Tang QL., Zhao S., Zhang WY., Xia QJ., Li GD. Real-time fluorescence quantitative PCR in detecting ribosome protein S13 (RPS13) gene expression in NK/T cell lymphoma. Sichuan Da Xue Xue Bao Yi Xue Ban. 37:464–466. 2006.
Yoshida H., Nozu F., Lankischo TO., Mitamura K., Owyang C., Tsunoda Y. A possible role for Ca(2+)/calmodulin-dependent protein kinase IV during pancreatic acinar stimulus-secretion coupling. Biochim Biophys Acta. 1497:155–167. 2000.

Yu JH., Lim JW., Kim H., Kim KH. NADPH oxidase mediates interukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 37:1458–1469. 2005.
Yu JH., Lim JW., Namkung W., Kim H., Kim KH. Suppression of cerulein-induced cytokine expression by antioxidants in pancreatic acinar cells. Lab Inv. 82:1359–1368. 2002.

Yu JH., Kim KH., Kim H. SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-bata by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol. 40:677–688. 2008.
Yu JH., Kim KH., Kim H. Suppression of IL-1beta expression by the Jak 2 inhibitor AG490 in cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol. 72:1555–1562. 2006.
Zhou W., Shen F., Miller JE., Han Q., Olson MS. Evidence of altered cellular calcium in the pathogenetic mechanism of acute pancreatitis in rats. J Surg Res. 60:147–155. 1996.
Go to : 
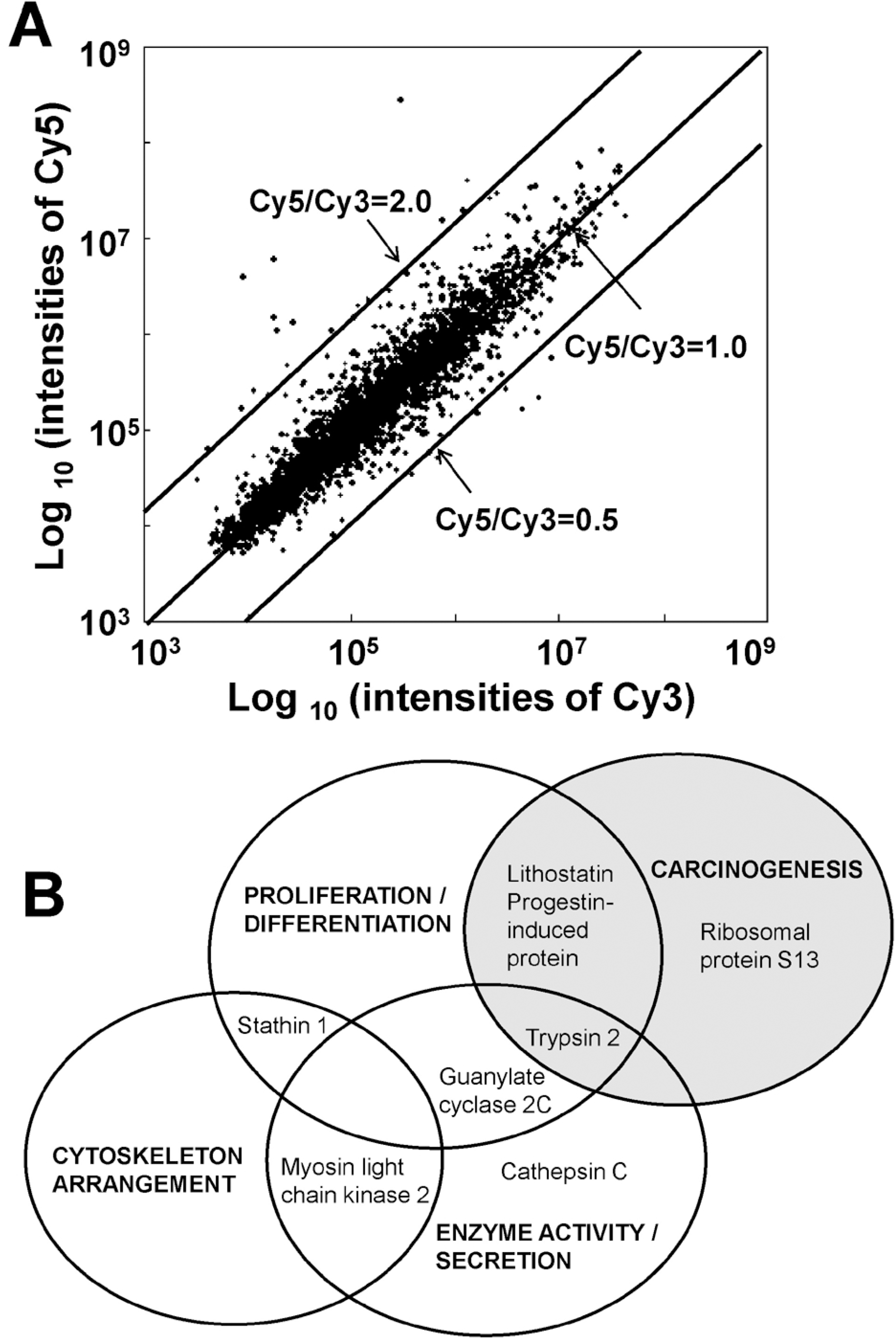 | Fig. 1.A representative scatter plot of cDNA microarray analysis and modified Venn diagram according to gene function. (A) AR42J cells stimulated with cerulein (labeled with Cy5) or without cerulein (labeled with Cy3) were labeled and hybridized to the cDNA microarray. Cy5/Cy3 ratios indicate relative expression levels. (B) Venn diagram of genes shows functional overlap. Cerulein changed genes related to cell proliferation and differentiation, carcinogenesis, enzyme activity and secretion and cytoskeleton arrangement. |
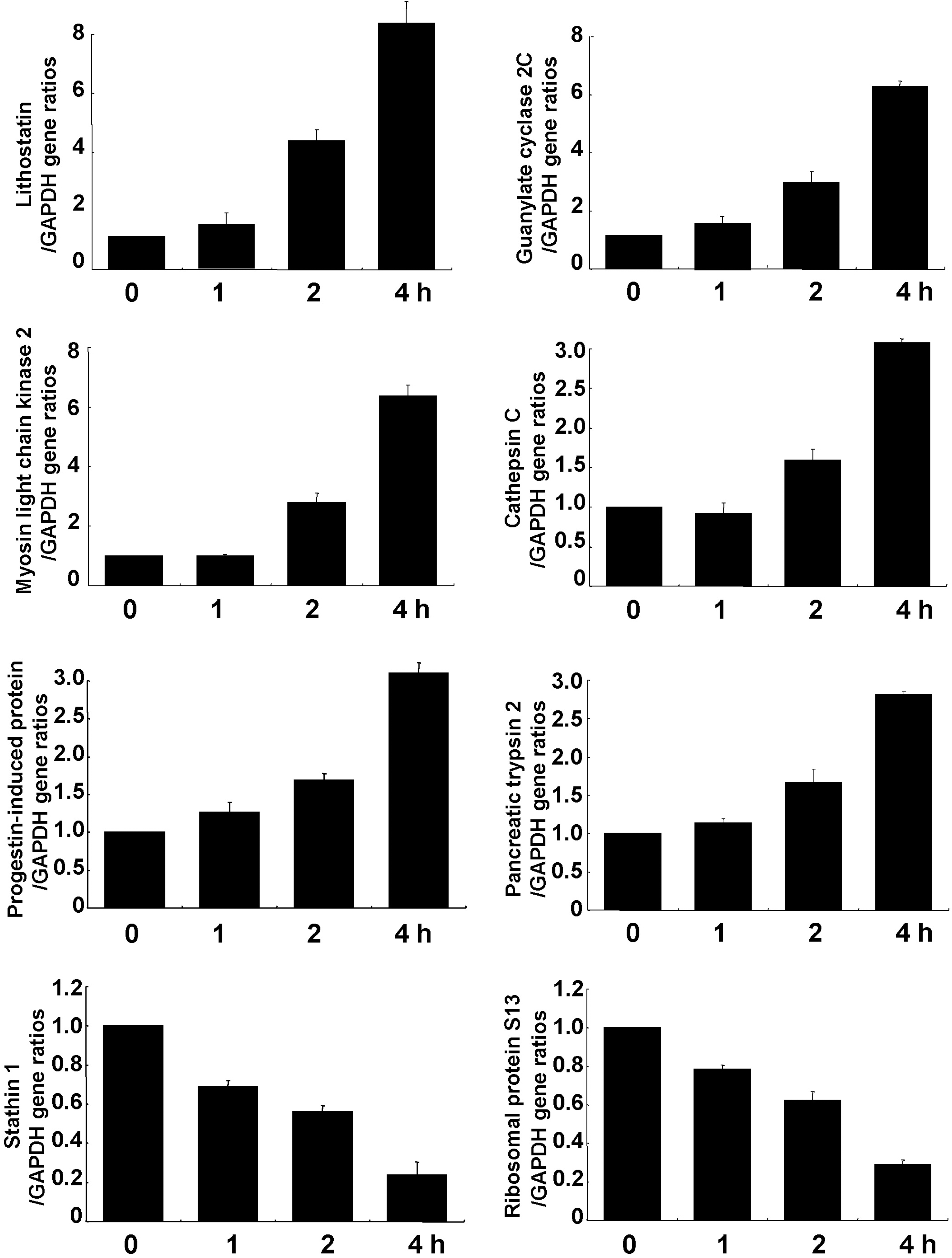 | Fig. 2.Time-dependent mRNA expression after cerulein treatment for 8 genes. Relative mRNA expression in AR42J cells treated with cerulein (10–8 M) was assessed by real-time RT-PCR. The internal standard (GAPDH) was coamplified with each gene. |
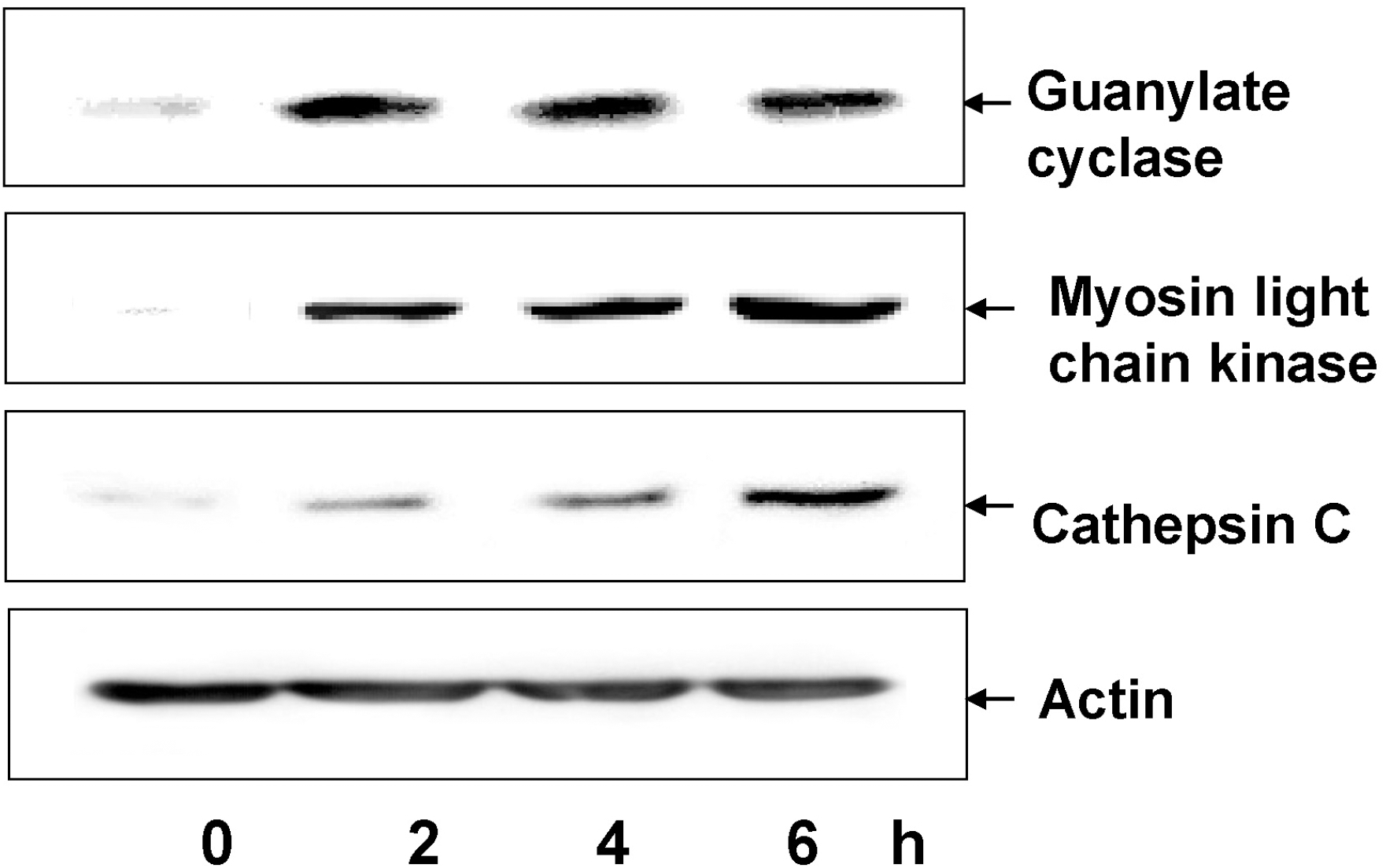 | Fig. 3.Western blot analysis for guanylate cyclase, myosin light chain kinase 2, and cathepsin C. Cells were cultured with cerulein for 6 h, harvested, lysed, and extracted. Whole cell extracts (50 μg of protein/lane) were loaded, separated by 8∼10% SDS-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes by electroblotting. The membranes were blocked with 5% nonfat dry milk in TBS-T. The proteins were detected with specific antibodies. After washing in TBS-T, the immunoreactive proteins were visualized using secondary antibodies conjugated to horseradish peroxidase, followed by enhanced chemiluminescence. Actin was used as a loading control. |
Table 1.
Altered genes by cerulein
| No. | Gene | Primer sequencesa | Foldb |
|---|---|---|---|
| Up-regulated genes | Cy5/Cy3 | ||
| 1 | Regeneration protein, lithostatin (Pancreatic stone protein) | (F) ACACCTTGTATCTGTGCTCAATGTAG | 7.86 |
| (R) CAAACTAAAGCTGTTTGCTGTCTGGTA | |||
| 2 | Guanylate cyclase 2C | (F) GTGACATTGTCGGTTTCACG | 6.13 |
| (R) CAAGGCCATCTTGGAAATGT | |||
| 3 | Myosin light chain kinase 2 | (F) CTGACAAGACGGACATGTGG | 5.71 |
| (R) AAGTCTTTGGCCTCGTCTGA | |||
| 4 | Cathepsin C | (F) TCAGACCCCAATCCTGAGTC | 3.76 |
| (R) AACGGAGGCAGTTTTCCTTT | |||
| 5 | Progestin-induced protein | (F) CTGGCAAAAACACAGAAGCA | 3.35 |
| (R) AGCATCGGCATCTGAACTCT | |||
| 6 | Pancreatic trypsin 2 | (F) GGAGGATACACCTGCCAAGA | 2.83 |
| (R) TCCTATCGAAGTTGGGATGC | |||
| Down-regulated genes | Cy3/Cy5 | ||
| 1 | Stathin 1 | (F) AAGGATCTTTCCCTGGAGGA | 2.66 |
| (R) TTCTCCTCTGCCATTTTGCT | |||
| 2 | Ribosomal protein S13 | (F) ACCGGCTGGCTCGATACTA | 2.50 |
| (R) GCTTGTGTACGCAACAGCAT |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download