Abstract
NO released by myenteric neurons controls the off contraction induced by electrical field stimulation (EFS) in distal esophageal smooth muscle, but in the presence of nitric oxide synthase (NOS) inhibitor, L-NAME, contraction by EFS occurs at the same time. The authors investigated the intracellular signaling pathways related with G protein and ionic channel EFS-induced contraction using cat esophageal muscles. EFS-induced contractions were significantly suppressed by tetrodotoxin (1 μM) and atropine (1 μM). Furthermore, nimodipine inhibited both on and off contractions by EFS in a concentration dependent meaner. The characteristics of ‘on’ and ‘off’ contraction and the effects of G-proteins, phospholipase, and K+ channel on EFS-induced contraction in smooth muscle were also investigated. Pertussis toxin (PTX, a Gi inactivator) attenuated both EFS-induced contractions. Cholera toxin (CTX, Gs inactivator) also decreased the amplitudes of EFS-induced off and on contractions. However, phospholipase inhibitors did not affect these contractions. Pinacidil (a K+ channel opener) decreased these contractions, and tetraethylammonium (TEA, K+Ca channel blocker) increased them. These results suggest that EFS-induced on and off contractions can be mediated by the activations Gi or Gs proteins, and that L-type Ca2+ channel may be activated by G-protein α subunits. Furthermore, K+Ca– channel involve in the depolarization of esophageal smooth muscle. Further studies are required to characterize the physiological regulation of Ca2+ channel and to investigate the effects of other K+ channels on EFS-induced on and off contractions.
Go to : 
References
Archer SL., Huang JM., Hampl V., Nelson DP., Shultz PJ., Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 91:7583–7587. 1994.

Behar J., Guenard V., Walsh JH., Biancani P. VIP and acetylcholine: neurotransmitters in esophageal circular smooth muscle. Am J Physiol. 257:G380–385. 1989.

Biancani P., Hillemeier C., Bitar KN., Makhlouf GM. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am J Physiol. 253:G760–766. 1987.
Biancani P., Walsh JH., Behar J. Vasoactive intestinal polypeptide. A neurotransmitter for lower esophageal sphincter relaxation. J Clin Invest. 73:963–967. 1984.

Bolotina VM., Najibi S., Palacino JJ., Pagano PJ., Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 368:850–853. 1994.

Carabelli V., Hernandez-Guijo JM., Baldelli P., Carbone E. Direct autocrine inhibition and cAMP-dependent potentiation of single L-type Ca2+ channels in bovine chromaffin cells. J Physiol. 532:73–90. 2001.
Casey PJ., Gilman AG. G protein involvement in receptor-effector coupling. J Biol Chem. 263:2577–2580. 1988.

Cayabyab FS., Daniel EE. K+ channel opening mediates hyper-polarizations by nitric oxide donors and IJPs in opossum esophagus. Am J Physiol. 268:G831–842. 1995.
Christinck F., Jury J., Cayabyab F., Daniel EE. Nitric oxide may be the final mediator of nonadrenergic, noncholinergic inhibitory junction potentials in the gut. Can J Physiol Pharmacol. 69:1448–1458. 1991.

Conklin JL., Du C. Guanylate cyclase inhibitors: effect on inhibitory junction potentials in esophageal smooth muscle. Am J Physiol. 263:G87–90. 1992.

Conklin JL., Du C., Murray JA., Bates JN. Characterization and mediation of inhibitory junction potentials from opossum lower esophageal sphincter. Gastroenterology. 104:1439–1444. 1993.

Crist J., Gidda JS., Goyal RK. Characteristics of ‘on’ and ‘off’ contractions in esophageal circular muscle. in vitro. Am J Physiol. 246:G137–144. 1984.
Dalziel HH., Thornbury KD., Ward SM., Sanders KM. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 260:G789–792. 1991.

Du C., Murray J., Bates JN., Conklin JL. Nitric oxide: mediator of NANC hyperpolarization of opossum esophageal smooth muscle. Am J Physiol. 261:G1012–1016. 1991.

Fleischmann BK., Murray RK., Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci USA. 91:11914–11918. 1994.

Goyal RK., Rattan S., Said SI. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 288:378–380. 1980.

Hillemeier C., Bitar KN., Marshall JM., Biancani P. Intracellular pathways for contraction in gastroesophageal smooth muscle cells. Am J Physiol. 260:G770–775. 1991.

Ivanina T., Blumenstein Y., Shistik E., Barzilai R., Dascal N. Modulation of L-type Ca2+ channels by gbeta gamma and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem. 275:39846–39854. 2000.
Jessell TM., Kandel ER. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 72(Suppl):1–30. 1993.

Miyoshi H., Nakaya Y. Endotoxin-induced nonendothelial nitric oxide activates the Ca2+-activated K+ channel in cultured vascular smooth muscle cells. J Mol Cell Cardiol. 26:1487–1495. 1994.
Murray J., Du C., Ledlow A., Bates JN., Conklin JL. Nitric oxide: mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 261:G401–406. 1991.

Murray JA., Shibata EF., Buresh TL., Picken H., O'Meara BW., Conklin JL. Nitric oxide modulates a calcium-activated potassium current in muscle cells from opossum esophagus. Am J Physiol. 269:G606–612. 1995.

Park SY., Shin CY., Song HJ., Min YS., La Hyen O., Lee JW., Kim do Y., Je HD., Sohn UD. Electrically stimulated relaxation is not mediated by GABA in cat lower esophageal sphincter muscle. Arch Pharm Res. 29:400–404. 2006.

Park SY., Song HJ., Sohn UD. Participation of Rho-associated kinase in electrical stimulated and acetylcholine-induced contraction of feline esophageal smooth muscle. Eur J Pharmacol. 607:220–225. 2009.

Rattan S., Chakder S. Role of nitric oxide as a mediator of internal anal sphincter relaxation. Am J Physiol. 262:G107–112. 1992.

Roman C. Nervous control of esophageal peristalsis. J Physiol (Paris). 58:79–108. 1966.
Roman C., Car A. Esophageal contractions produced by stimulation of the vagus or of the medulla oblongata. J Physiol (Paris). 59:377–398. 1967.
Sato K., Sanders KM., Gerthoffer WT., Publicover NG. Sources of calcium utilized in cholinergic responses in canine colonic smooth muscle. Am J Physiol. 267:C1666–1673. 1994.

Sims SM., Jiao Y., Preiksaitis HG. Regulation of intracellular calcium in human esophageal smooth muscles. Am J Physiol. 273:C1679–1689. 1997.
Sohn UD., Chiu TT., Bitar KN., Hillemeier C., Behar J., Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol. 266:G330–338. 1994.

Sohn UD., Han B., Tashjian AH Jr., Behar J., Biancani P. Agonist-independent, muscle-type-specific signal transduction pathways in cat esophageal and lower esophageal sphincter circular smooth muscle. J Pharmacol Exp Ther. 273:482–491. 1995.
Sohn UD., Harnett KM., De Petris G., Behar J., Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 267:1205–1214. 1993.
Somlyo AP., Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 372:231–236. 1994.

Tottrup A., Svane D., Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol. 260:G385–389. 1991.

Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell. 72(Suppl):31–41. 1993.

van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 51:315–329. 1989.
Viard P., Exner T., Maier U., Mironneau J., Nurnberg B., Macrez N. Gbetagamma dimers stimulate vascular L-type Ca2+ channels via phosphoinositide 3-kinase. Faseb J. 13:685–694. 1999.
Wade GR., Laurier LG., Preiksaitis HG., Sims SM. Delayed rectifier and Ca2+-dependent K+ currents in human esophagus: roles in regulating muscle contraction. Am J Physiol. 277:G885–895. 1999.
Weisbrodt NW., Christensen J. Gradients of contractions in the opossum esophagus. Gastroenterology. 62:1159–1166. 1972.

Yamato S., Saha JK., Goyal RK. Role of nitric oxide in lower esophageal sphincter relaxation to swallowing. Life Sci. 50:1263–1272. 1992.

Yang SJ., An JY., Shim JO., Park CH., Huh IH., Sohn UD. The mechanism of contraction by 2-chloroadenosine in cat detrusor muscle cells. J Urol. 163:652–658. 2000.

Zhong J., Dessauer CW., Keef KD., Hume JR. Regulation of L-type Ca2+ channels in rabbit portal vein by G protein alphas and betagamma subunits. J Physiol. 517:109–120. 1999.
Go to : 
 | Fig. 1.Identification of ‘on’ and ‘off’ contraction. (A) ‘Off’ contractions were evoked by electrical field stimulation (EFS) in esophageal circular smooth muscle. (B) L-NAME (100 μM, a NOS inhibitor) was pretreated for 10 min, thus the contractions was provoked during EFS (‘On’ contractions). |
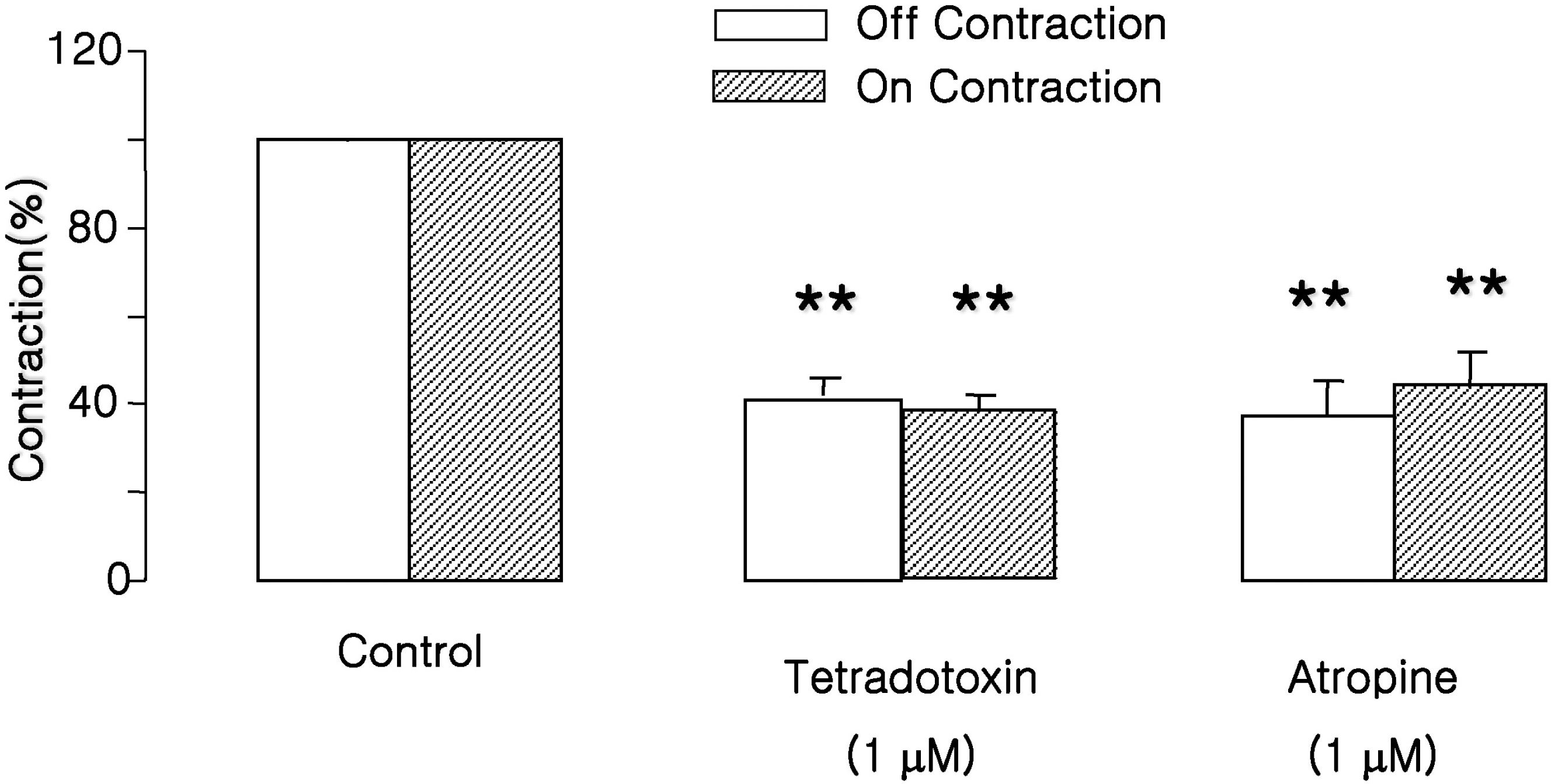 | Fig. 2.Effects of atropine and tetrodotoxin on EFS-induced contractions. ‘On’ and ‘off’ contractions were abolished by tetrodotoxin (1 μM, administered 15 min before EFS) and atropine (1 μM, 15 min before EFS). Values are expressed as means± SEM. ∗∗p<0.01 versus control value. |
 | Fig. 3.Effect of Ca2+ blocker on EFS-induced contractions. Nimodipine (an L-type Ca2+ channel blocker) inhibited EFS-induced ‘off’ and ‘on’ contractions in a concentration-dependent manner, which suggested that Ca2+ channel contributes to the activation of muscle contraction in response to EFS. Values are expressed as means± SEM. ∗p<0.05, ∗∗p<0.01 versus untreated controls. |
 | Fig. 4.Effects of PTX (A) and CTX (B) on response to EFS. EFS-induced contractions were significantly inhibited by PTX (800 ng/ml, a Gi inactivator, n=5) after 2 hr of pretreatment, which indicated that Gi protein mediated EFS-induced muscle contraction. Furthermore, EFS-induced contractions were also significantly inhibited by CTX (2 μg/ml, Gs inactivator, n=5), which indicated that Gs protein mediated EFS-induced muscle contraction. Values are expressed as means± SEM. ∗∗p<0.01 versus untreated controls. |
 | Fig. 5.Effects of phospholipases inhibitors on EFS-induced contractions. D609 (10 μM, a phosphatidylcholine-PLC inhibitor), pCMB (10 μM, a PLD inhibitor), U73122 (10 μM, a phosphatidylinositol-PLC inhibitor), and DEDA (10 μM, a phospholipase A2) had no effect on EFS-induced (A) ‘off’ or (B) ‘on’ contractions. Values are expressed as means± SEMs. |
 | Fig. 6.Effect of a K+ Channel opener on EFS-induced contractions. Pinacidil (a K+ Channel opener) decreased the amplitude of both ‘on’ and ‘off’ EFS-induced contractions, which suggested that K+ efflux through K+ channel reduced response to EFS. Values are expressed as means±SEMs. ∗p<0.05, ∗∗p<0.01 versus untreated controls. |
 | Fig. 7.Effect of K+ca- Channel Blocker on response to EFS. EFS-induced ‘off’ and ‘on’ contractions were found to be augmented by TEA (a Ca2+-dependent K+ channel blocker). Values are expressed as means±SEMs. ∗p<0.05, ∗∗p<0.01 versus untreated controls. |
 | Fig. 8.The signaling diagram induced by EFS in cat esophageal muscle. The ‘off’ contraction (A) is probably mediated via NO-mediated G kinase dependent relaxation. Interestingly, our findings suggest that ‘on’ and ‘off’ (B) contractions are mediated by Gi/s proteins via the opening of L-type Ca2+ channel, and that these contractions are augmented by the blocking of Ca2+-dependent K+ channel. However, it remains to be determined which muscarinic receptor and whether contractile proteins and intracellular Ca2+ stores are involved in EFS-induced ‘on’ and ‘off’ contraction. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download