Abstract
Cerebellar Purkinje cells (PCs) play a crucial role in motor functions and their progressive degeneration is closely associated with spinocerebellar ataxias. Although immunohistochemical (IHC) analysis can provide a valuable tool for understanding the pathophysiology of PC disorders, the method validation of IHC analysis with cerebellar tissue specimens is unclear. Here we present an optimized and validated IHC method using antibodies to calbindin D28k, a specific PC marker in the cerebellum. To achieve the desired sensitivity, specificity, and reproducibility, we modified IHC analysis procedures for cerebellar tissues. We found that the sensitivity of staining varies depending on the commercial source of primary antibody. In addition, we showed that a biotin-free signal amplification method using a horseradish peroxidase polymer-conjugated secondary antibody increases both the sensitivity and specificity of ICH analysis. Furthermore, we demonstrated that dye filtration using a 0.22 μm filter eliminates or minimizes nonspecific staining while preserving the analytical sensitivity. These results suggest that our protocol can be adapted for future investigations aiming to understand the pathophysiology of cerebellar PC disorders and to evaluate the efficacy of therapeutic strategies for treating these diseases.
Go to : 
References
Airaksinen MS., Eilers J., Garaschuk O., Thoenen H., Konnerth A., Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA. 94:1488–1493. 1997.

Alon R., Bayer EA., Wilchek M. Cell adhesion to streptavidin via RGD-dependent integrins. Eur J Cell Biol. 60:1–11. 1993.
Alon R., Bayer EA., Wilchek M. Cell-adhesive properties of streptavidin are mediated by the exposure of an RGD-like RYD site. Eur J Cell Biol. 58:271–279. 1992.
Alon R., Bayer EA., Wilchek M. Streptavidin contains an RYD sequence which mimics the RGD receptor domain of fibronectin. Biochem Biphys Res Commun. 179:1236–1241. 1990.

Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2:242–262. 2003.

Cheron G., Servais L., Dan B. Cerebellar network plasticity: from genes to fast oscillation. Neuroscience. 153:1–19. 2008.

Dapson RW. Dye-tissue interactions: mechanisms, quantification and bonding parameters for dyes used in biological staining. Biotech Histochem. 80:49–72. 2005.

Douyard J., Shen L., Huganir RL., Rubio ME. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1 N during rat cerebellar development. J Comp Neurol. 502:141–156. 2007.
Fukuda T., Tani Y., Kobayashi T., Hirayama Y., Hino O. A new Western blotting method using polymer immunocomplexes: detection of Tsc1 and Tsc2 expression in various cultured cell lines. Anal Biochem. 285:274–276. 2000.

Goldstein NS., Hewitt SM., Taylor CR., Yaziji H., Hicks DG. Members of Ad-Hoc Committee On Immunohistochemistry Standardization, Recommendations for improved standardization of immuno-histochemistry. Appl Immunohistochem Mol Morphol. 15:123–133. 2007.
Green M., Sviland L., Taylor CE., Peiris M., McCarthy . al., Pearson AD, Malcolm AJ. Human herpes virus 6 and endogenous biotin in salivary glands. J Clin Pathol. 45:788–90. 1992.
Haworth R., McCormack N., Selway S., Pilling AM., Williams TC. Calbindin D-28 and microtubule-associated protein-2: Their use as sensitive immunohistochemical markers of cerebellar neurotoxicity in a regulatory toxicity study. Exp Toxicol Pathol. 57:419–426. 2006.

Iezzoni jc., Mills SE., Pelkey TJ., Stoler MH. Inhibin is not an immunohistochemical marker for hepatocellular carcinoma. An example of the potential pitfall in diagnostic immunohistochemistry caused by endogenous biotin. Am J Clin Pathol. 111:229–234. 1999.

Ito M. Historical review of the significance of the cerebellum and the role of Purkinje neurons in motor learning. Ann NY Acad Sci. 978:273–288. 2002.
Ivan GJ. Synaptic signaling in cerebellar plasticity. Biol Cell. 99:363–378. 2007.
Jeon JH., Kim CW., Shin DM., Cho SY., Jang GY., Lee HJ., Kim IG. Colorimetric transglutaminase assays combined with immunological signal amplification. Anal Biochem. 348:327–329. 2006.

Jeon JH., Shin DM., Cho SY., Song KY., Park NH., Kang HS., Kim YD., Kim IG. Immunocytochemical detection of HPV16 E7 in cervical cancer. Exp Mol Med. 39:621–628. 2007.
Lyon HO. Dye purity and dye standardization for biological staining. Biotech Histochem. 77:57–80. 2002.

Millen KJ. Gleeson JG, Cerebellar development and disease. Curr Opin Neurobiol. 18:12–19. 2008.
Nakamura R., Kurita K., Kawanami T., Kato T. An immunohistochemical study of Purkinje cells in a case of hereditary cerebellar cortical atrophy. Act Neuropathol. 97:196–200. 1999.

O'Leary TJ. Standardization in immunohistochemistry. Appl Immunohistochem Mol Morphol. 9:3–8. 2001.
Schwaller B., Meyer M., Schiffmann S. New function for old proteins: the role of the calcium-binding proteins Calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 1:241–258. 2002.
Simons MJ., Pellionisz AJ. Genomics, morphogenesis and biophysics: Triangulation of Purkinje cell development. Cerebellum. 5:27–35. 2006.

Sotelo C. Cellular and genetic regulaton of the development of the cerebellar system. Prog Neurobiol. 72:295–339. 2004.
Whitney ER., Kemper TL., Bauman ML., Rosene DL., Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin D-28k. Cerebellum. 7:406–416. 2008a.
Whitney ER., Kemper TL., Rosene DL., Bauman ML., Blatt GJ. Calbindin-D28k is a more reliable marker of human Purkinje cells that standard Nissl stains: a stereological experiment. J Neurosci Methods. 168:42–47. 2008b.
Go to : 
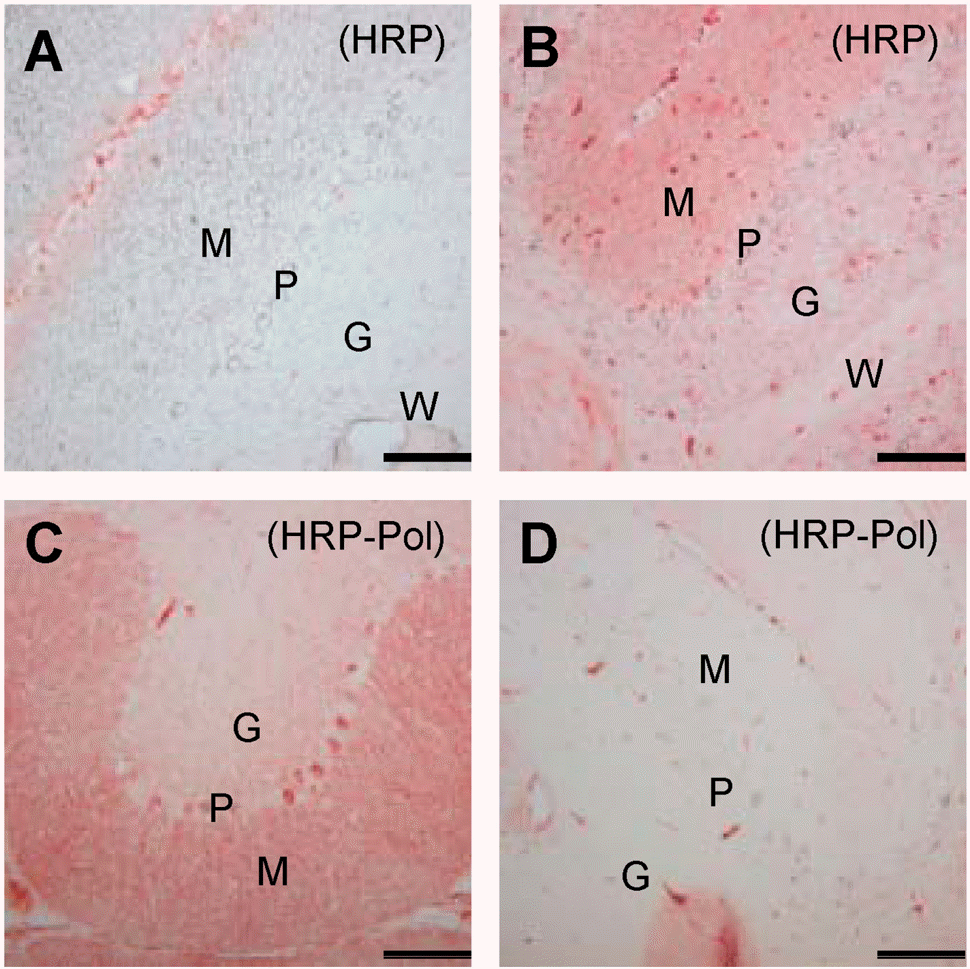 | Fig. 1.Sensitivity and specificity of IHC methods. The expression of CaB in cerebellar specimens was analyzed by the HRP method without (A) or with (B) 1:500-diluted anti-CaB antibody (Sigma) and stained using unfiltered AEC solution. CaB was captured by two different commercial source antibodies, one from Sigma (C) and another from Chemicon (D), for 1 h at RT. Antibodies were probed by HRP polymer-conjugated secondary antibody (HRP-Pol). M, molecular layer; P, Purkinje cell layer; G, granular cell layer; W, white matter region. Scale bar=100 μm. The data shown are representative results of three to five independent experiments. |
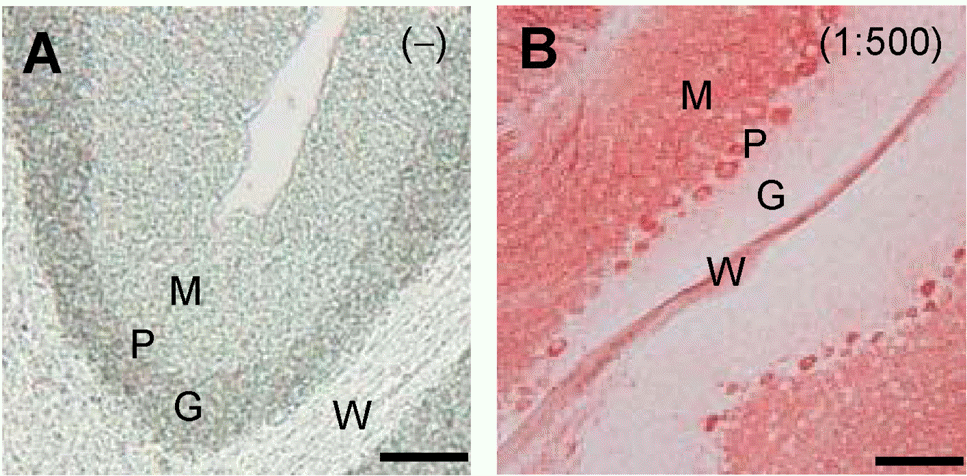 | Fig. 2.Increased sensitivity of IHC analysis by filtrating AEC solution. CaB expression in cerebellar specimens was analyzed by the HRP-Pol method without (A) or with (B) 1:500-diluted anti-CaB antibody (Sigma). AEC solution was filtrated with a 0.22 μm syringe filter before adding to tissue specimens. Scale bar=100 μm. The data shown are representative results of three to five independent experiments. |
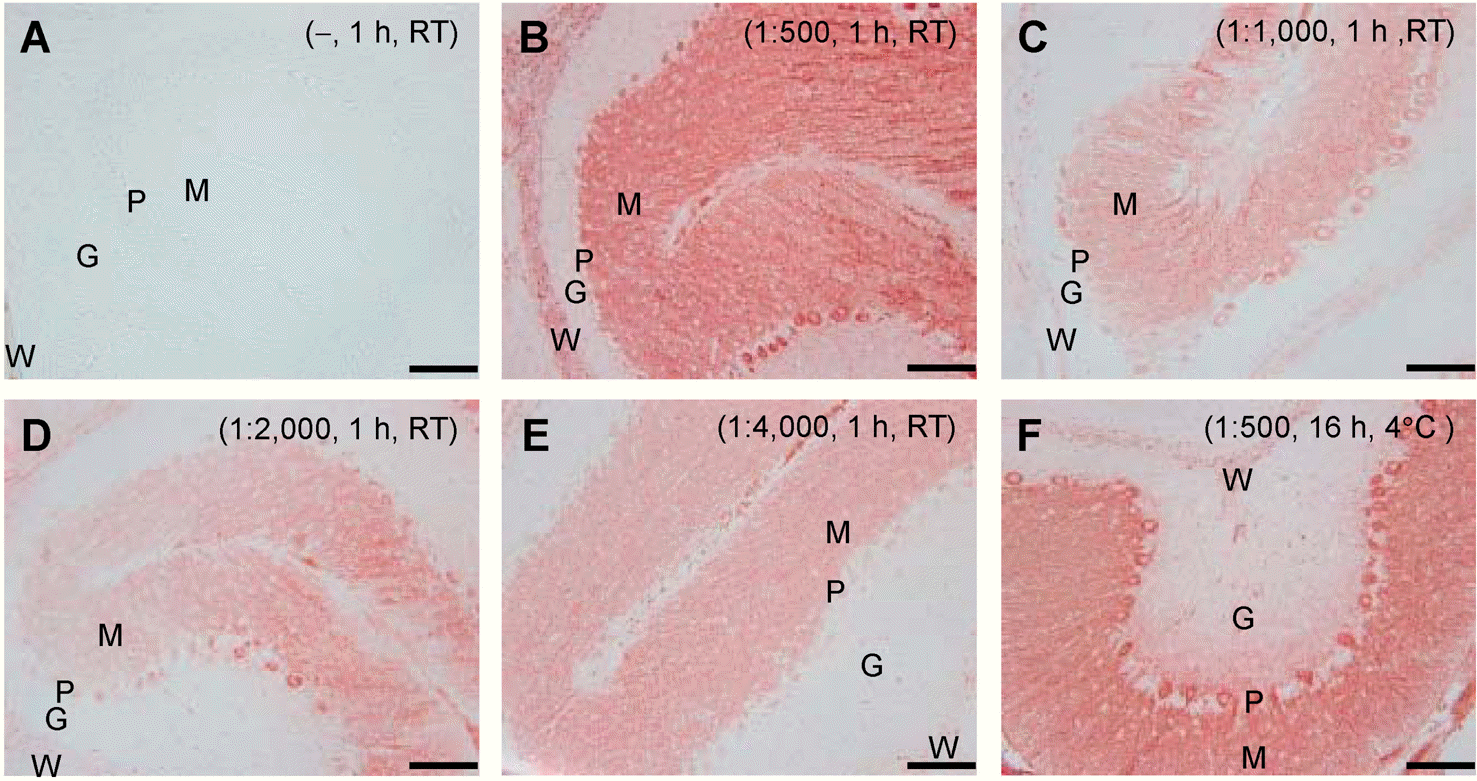 | Fig. 3.Determination of the optimal titer of primary antibody. Serially diluted primary antibodies to CaB were used to determine the optimum titer (B∼E). The HRP-Pol method with filtrated dye was employed for IHC analysis. Staining specificity was verified by omitting the incubation step with primary antibody (A). Cerebellar specimens were incubated with primary antibody for 1 h at RT (A∼E) or for 16 h at 4°C (F). Scale bar=100 μm. The data shown are representative results of three to five independent experiments. |
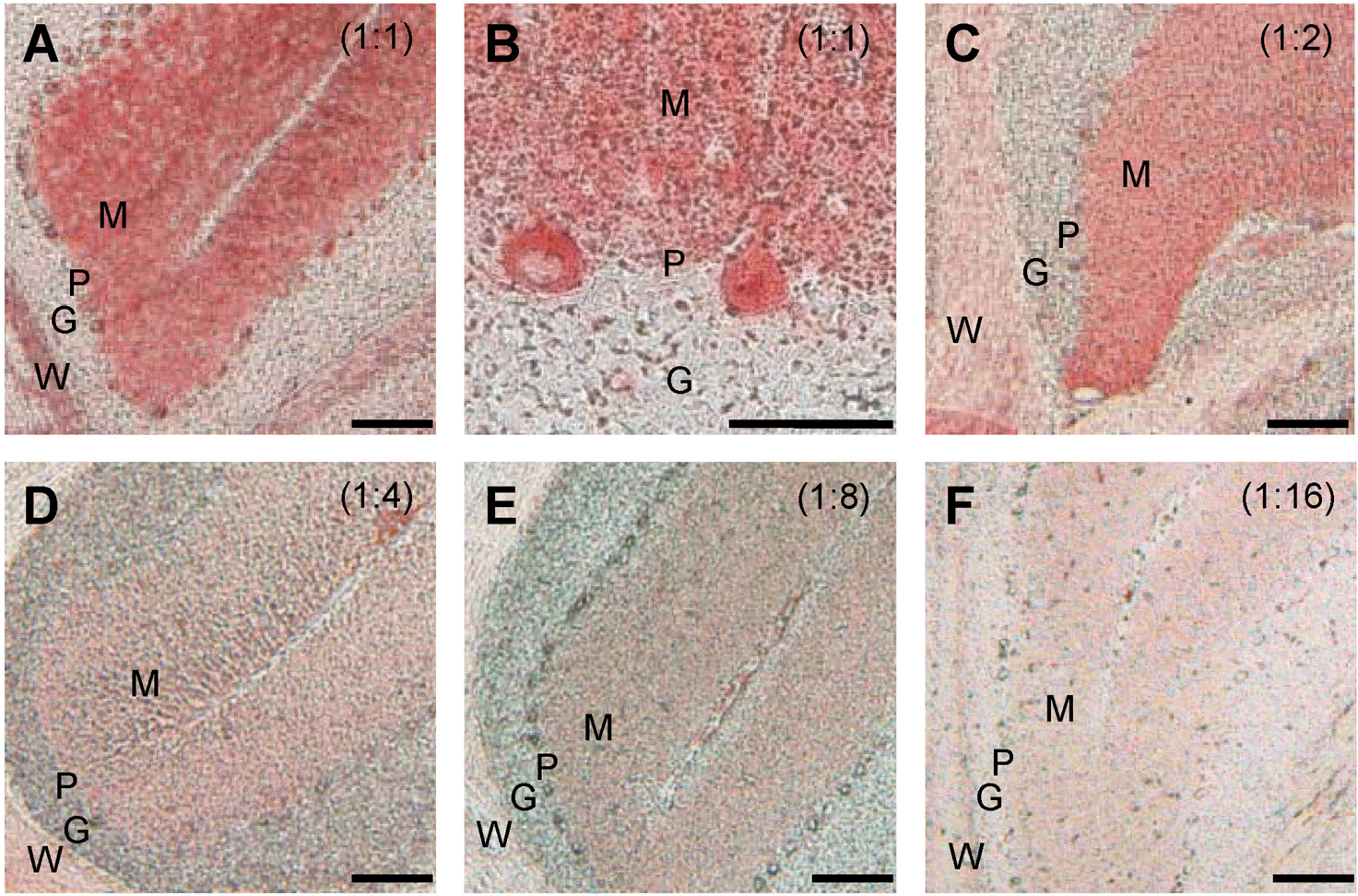 | Fig. 4.Determination of the optimal titer of HRP polymer-conjugated secondary antibody. Serially diluted HRP polymer-conjugated secondary antibodies were used to determine the optimum titer (A∼F). The cerebellar specimens were incubated with primary antibody to CaB for 1 h at RT. Scale bar=100 μm. The experimental data shown are representative results of three to five independent experiments. |
Table 1.
Proposed IHC analysis protocol




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download