Abstract
It is hypothesized that a number of environmental factors affect animals' behavior. Without controlling these variables, it is very hard for researchers to get not only reliable, but replicable data from various behavioral experiments testing animals' cognitive as well as emotional functions. For example, laboratory mice which had restricted environment showed different synaptic potentiation properties with wild mice (Zhao MG et al., 2009). While performing behavioral experiments, however, it is sometimes inevitable that the researcher changes the animals' environments, as by switching the cages in which experimental animals are housed and separating animals raised together into small experimental groups. In this study, we investigated the effect of environmental changes on mice's emotional behaviors by socially isolating them or reducing the size of their cage. We found that social isolation selectively increases the animals' levels of anxiety, while leaving depression-like behaviors unchanged. On the other hand, alteration of the housing dimensions affected neither their anxiety levels nor their depression-like behaviors. These results suggest that environmental variables may have a prominent impact on experimental animals' emotional behaviors and possibly their psychological states, leading to bias in the behavioral data produced from experiments.
Go to : 
References
Chourbaji S., Zacher C., Sanchis-Segura C., Spanagel R., Gass P. Social and structural housing conditions influence the development of a depressive-like phenotype in the learned helplessness paradigm in male mice. Behav Brain Res. 164:100–106. 2005.

Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc. 1:936–946. 2006.

Hattori S., Hashimoto R., Miyakawa T., Yamanaka H., Maeno H., Wada K., Kunugi H. Enriched environments influence depression-related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav Brain Res. 180:69–76. 2007.

Karolewicz B., Paul IA. Group housing of mice increases immobility and antidepressant sensitivity in the forced swim and tail suspension tests. Eur J Pharmacol. 415:197–201. 2001.

Kirbya LG., Panb YZ., Freeman-Danielsa E., Rania S., Nunana JD., Akanwab A., Beckb SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 32:712–723. 2007.

Malberg JE., Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 28:1562–1571. 2003.

Millstein RA., Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 31:3–17. 2007.

Porsolt RD., Anton G., Blavet N., Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 4:379–391. 1978.

Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 463:3–33. 2003.

Go to : 
 | Fig. 1.Experimental design. (A) Procedure of behavior experiment. (B) Experimental groups. LtL, large to large cage; LtS, large to small cage; StS, small to small cage; LtiS, large to small cage, isolated; StiS, small to small cage, isolated. |
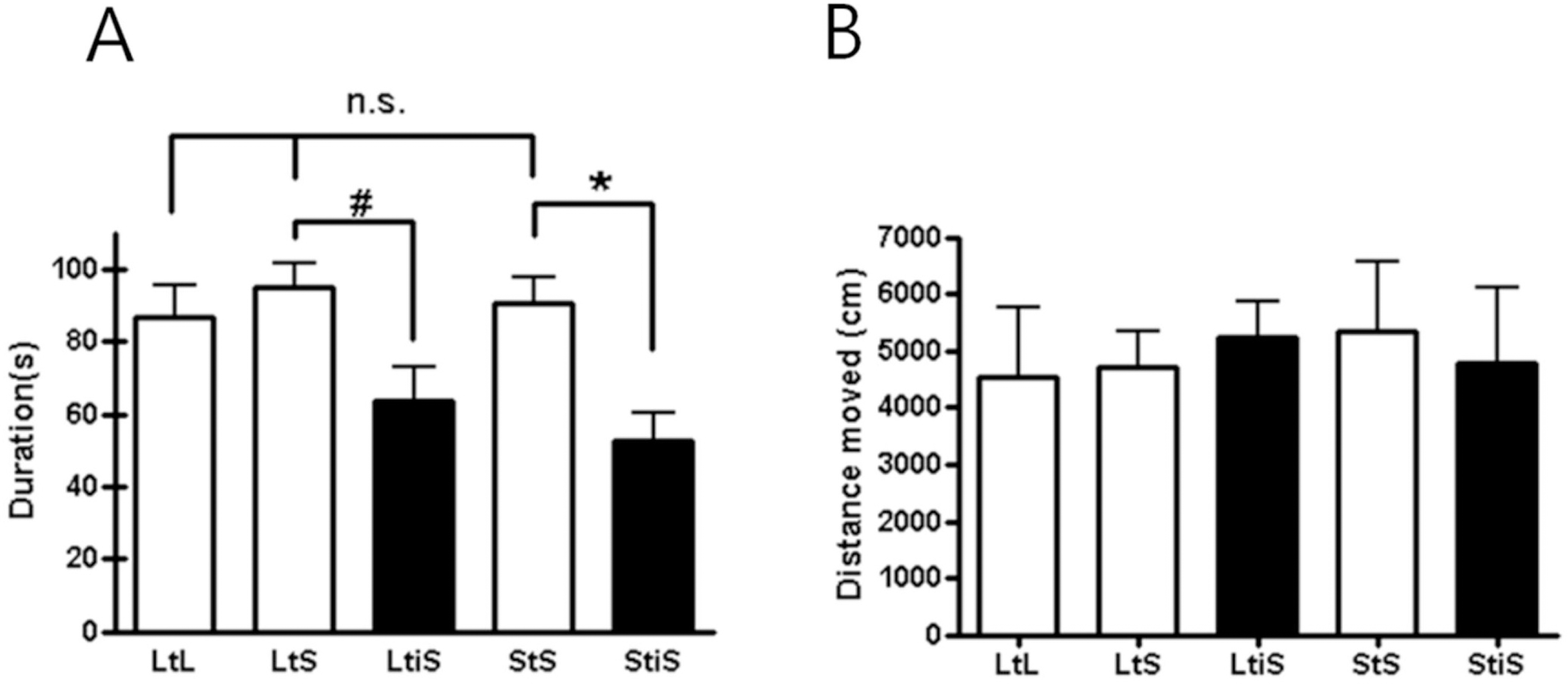 | Fig. 2.(A) The result of the open field (OF) test indicates that social isolation induced increasing anxiety levels (LtS vs. LtiS; LtS, 94.73±10.37 s, n=6; LtiS, 63.37±14.15 s, n=6; unpaired t-test, #p= 0.1041; StS vs. StiS; StS, 90.70±10.55 s, n=9; StiS, 52.53±11.03 s, n=6; unpaired t-test, ∗p=0.0317). (B) Not all mice showed different locomotion activity levels (one-way ANOVA, p=0.5529). |
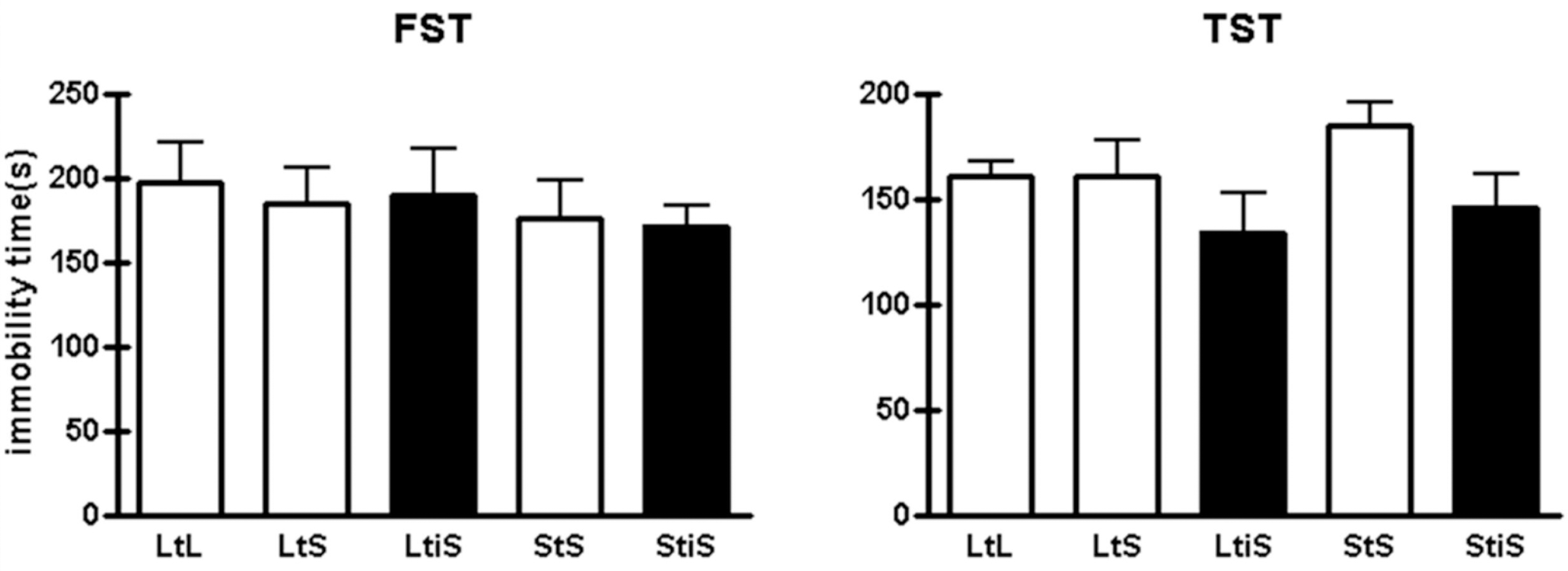 | Fig. 3.In the tail suspension test (TST) and forced swim test (FST), groups didn't show notably different levels of depression-like behavior among groups (TST: LtL, 160.33± 7.38 s, n=6; LtS, 160.20±17.32 s, n=5; LtiS, 134.00±18.59 s, n=6; StS, 185.00± 9.10 s; StiS, 146.11±15.21 s, n=9; oneway ANOVA, p=0.3152. FST: LtL, 197.50±23.42 s, n=6; LtS, 184.16± 21.94 s; LtiS, 189.67±28.00 s, n=6; StS, 176.17±22.65 s, n=6; StiS, 171.33± 11.85 s, n=9; one-way ANOVA, p=0.8973). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download