Abstract
Signal transducer and activator of transcription 4 (STAT4), a STAT family member, mediates interleukin 12 (IL12) signal transduction. IL12 is known to be related to calorie-restricted status. In the central nervous system, IL12 also enhances the production of nitric oxide (NO), which regulates food intake. In this study, the expression of neuronal NO synthase (Nos1), which is also related to food intake, was investigated in the hypothalamic areas of Stat4 knockout (KO) mice using nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) histochemistry, a marker for neurons expressing Nos1 enzyme. Western blots were also performed to evaluate Nos1 and Fos expression. Wild-type Balb/c (WT group, n=10 male) and Stat4 KO mice (Stat4 KO group, n=8 male) were used. The body weight and daily food intake in the WT group were 22.4±0.3 and 4.4 g per day, while those in the Stat4 KO group were 18.7±0.4 and 1.8 g per day, respectively. Stat4 mice had lower body weight and food intake than Balb/c mice. Optical intensities of NADPH-d-positive neurons in the paraventricular nucleus (PVN) and lateral hypothalamic area (LHA) of the Stat4 KO group were significantly higher than those of the WT group. Western blotting analysis revealed that the hypothalamic Nos1 and Fos expression of the Stat4 KO group was up-regulated, compared to that in the WT group. These results suggest that Stat4 may be related to the regulation of food intake and expression of Nos1 in the hypothalamus.
References
Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 31:577–596. 1999.

Copeland NG., Gilbert DJ., Schindler C., Zhong Z., Wen Z., Darnell JE Jr., Mui AL., Miyajima A., Quelle FW., Ihle JN., Jenkins NA. Distribution of the mammalian Stat gene family in mouse chromosomes. Genomics. 29:225–228. 1995.

Darnell JE Jr., Kerr IM., Stark GM. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 264:1415–1421. 1994.

Dawson TM., Dawson VL., Snyder SH. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 32:297–311. 1992.

Fenton JI., Nuñez NP., Yakar S., Perkins SN., Hord NG., Hursting SD. Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes Metab. 11:343–354. 2009.

Frucht DM., Aringer M., Galon J., Danning C., Brown M., Fan S., Centola M., Wu CY., Yamada N., El Gabalawy H., O'Shea JJ. Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J Immunol. 164:4659–4664. 2000.

Hoey T., Zhang S., Schmidt N., Yu Q., Ramchandani S., Xu X., Naeger LK., Sun YL., Kaplan MH. Distinct requirements for the naturally occurring splice forms Stat4alpha and Stat4beta in IL-12 responses. EMBO J. 22:4237–4248. 2003.
Kaplan MH., Sun YL., Hoey T., Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 382:174–177. 1996.

Kataoka TR., Komazawa N., Morii E., Oboki K., Nakano T. Involvement of connective tissue-type mast cells in Th1 immune responses via Stat4 expression. Blood. 105:1016–1020. 2005.

Kim MJ., Kim Y., Choe BK., Kim SA., Lee HJ., Kim JW., Huh Y., Kim C., Chung JH. Differential expression of nicotinamide adenine dinucleotide phosphate-diaphorase in hypothalamic areas of obese Zucker rats. Neurosci Lett. 292:60–62. 2000.

Min SS. Effects of nos inhibitors on arthritis and arthritic pain in rats. Korean J Physiol Pharmacol. 11:253–257. 2007.
Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 145:201–227. 1992.
Moncada S., Palmer RM., Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 43:109–142. 1991.
Morley JE., Flood JF. Competitive antagonism of nitric oxide synthase causes weight loss in mice. Life Sci. 51:1285–1289. 1992.
Morley JE., Flood JF. Effect of competitive antagonism of NO synthase on weight and food intake in obese and diabetic mice. Am J Physiol. 266:R164–168. 1994.
Morley JE., Flood JF. Evidence that nitric oxide modulates food intake in mice. Life Sci. 49:707–711. 1991.

Morley JE., Mattammal MB. Nitric oxide synthase levels in obese Zucker rats. Neurosci Lett. 209:137–139. 1996.

Raman K., Kaplan MH., Hogaboam CM., Berlin A., Lukacs NW. STAT4 signal pathways regulate inflammation and airway physiology changes in allergic airway inflammation locally via alteration of chemokines. J Immunol. 170:3859–3865. 2003.

Reiss CS., Komatsu T., Barna M., Bi Z. Interleukin-12 promotes enhanced recovery from viral infection of neurons in the central nervous system. Ann NY Acad Sci. 795:257–265. 1996.

Scherer-Singler U., Vincent SR., Kimura H., McGeer EG. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods. 9:229–234. 1983.

Squadrito F., Calapai G., Altavilla D., Cucinotta D., Zingarelli B., Campo GM., Arcoraci V., Sautebin L., Mazzaglia G., Caputi AP. Food deprivation increases brain nitric oxide synthase and depresses brain serotonin levels in rats. Neuropharmacology. 33:83–86. 1994.

Squadrito F., Calapai G., Cucinotta D., Altavilla D., Zingarelli B., Ioculano M., Urna G., Sardella A., Campo GM., Caputi AP. Anorectic activity of NG-nitro-L-arginine, an inhibitor of brain nitric oxide synthase, in obese Zucker rats. Eur J Pharmacol. 230:125–128. 1993.

Thierfelder WE., van Deursen JM., Yamamoto K., Tripp RA., Sarawar SR., Carson RT., Sangster MY., Vignali DA., Doherty PC., Grosveld GC., Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 382:171–174. 1996.

Yang EK. Role of angiotensin II and nitric oxide in the rat paraventricular nucleus. Korean J Physiol Pharmacol. 5:41–46. 2001.
Fig. 1.
Body weight in wild-type Balb/c and Stat4 knockout (KO) mice. Values are mean±SEM. ∗p<0.05 vs. Balb/c mice.
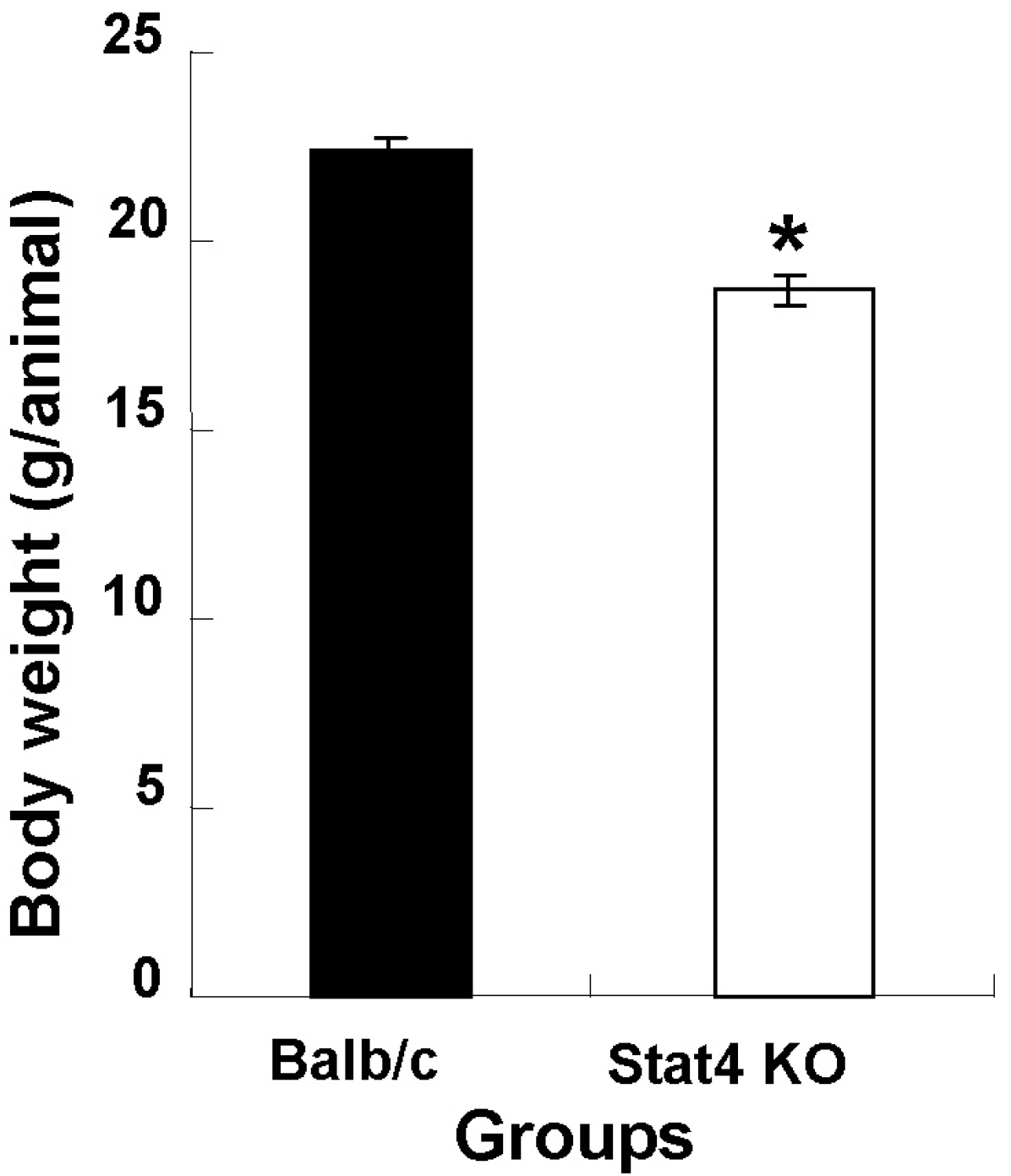
Fig. 2.
Daily food intake in wild-type Balb/c and Stat4 knockout (KO) mice. Values are mean±SEM. ∗p<0.05 vs. Balb/c mice.
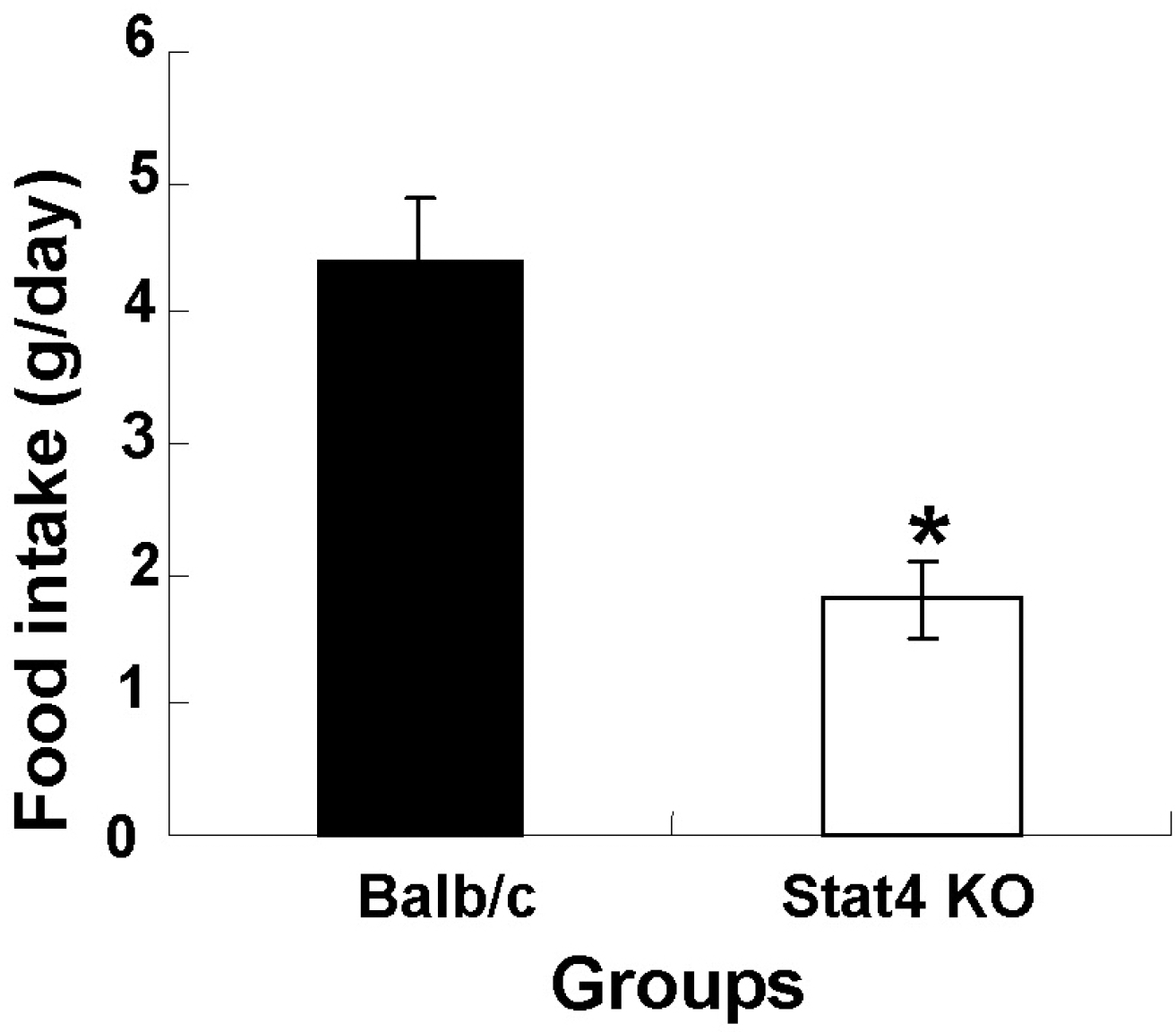
Fig. 3.
Distribution of NADPH-diaphorase-positive neurons in the hypothalamic regions. (A) The paraventricular nucleus of Balb/c mice (WT group). (B) The paraventricular nucleus of Stat4 knockout mice (Stat4 KO group). (C) The lateral hypothalamic area of the WT group. (D) The lateral hypothalamic area of the Stat4 KO group. Scale bar represents 80 μm.
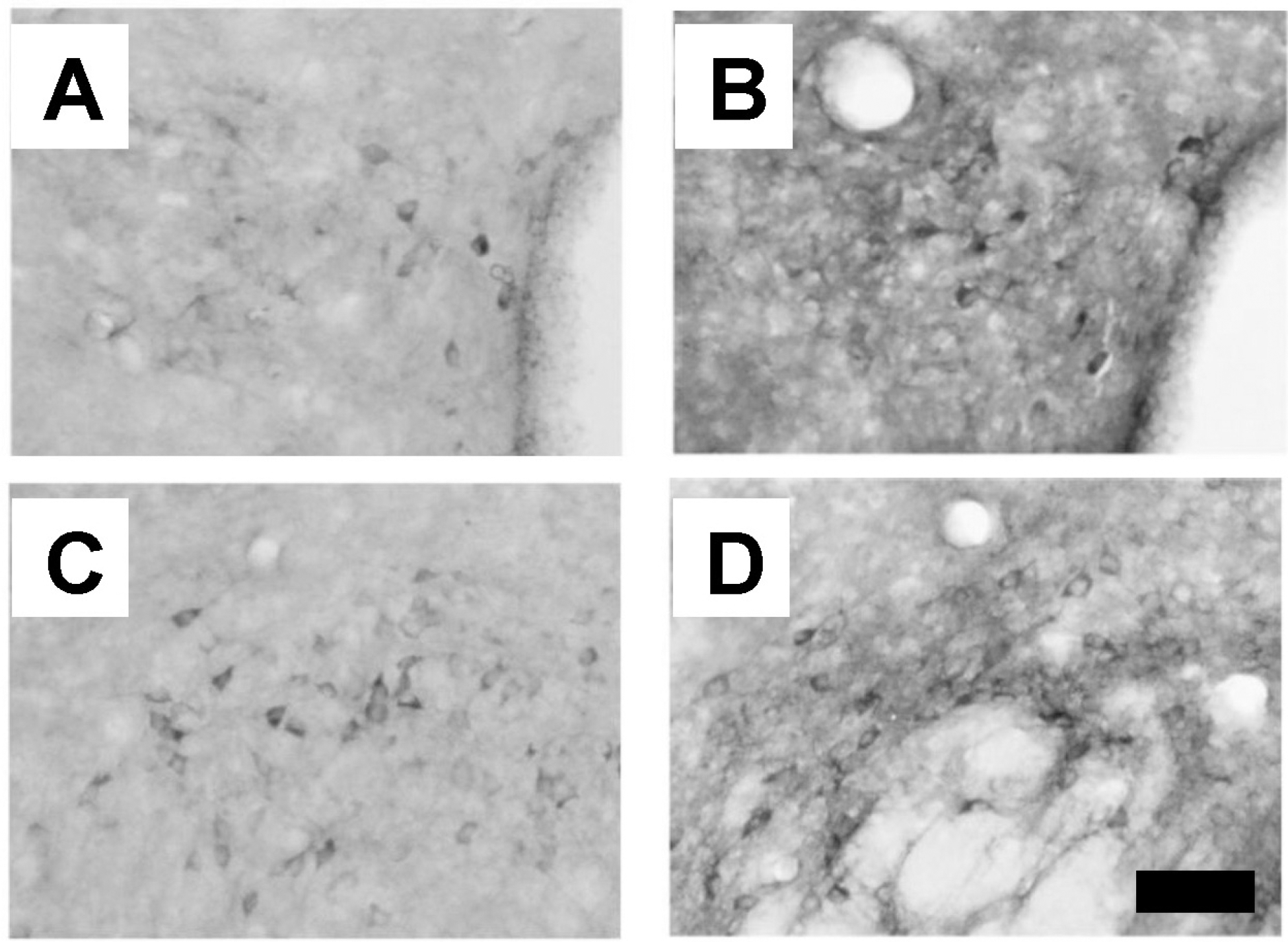
Fig. 4.
Optical density of NADPH-diaphorase-positive neurons in the hypothalamic regions. Values are mean±SEM. NADPH-d, nicotinamide adenine dinucleotide phosphate-diaphorase; PVN, paraventricular nucleus; LHA, lateral hypothalamic area; Stat4 KO, Stat4 knockout mice. ∗p<0.05 vs. Balb/c mice.
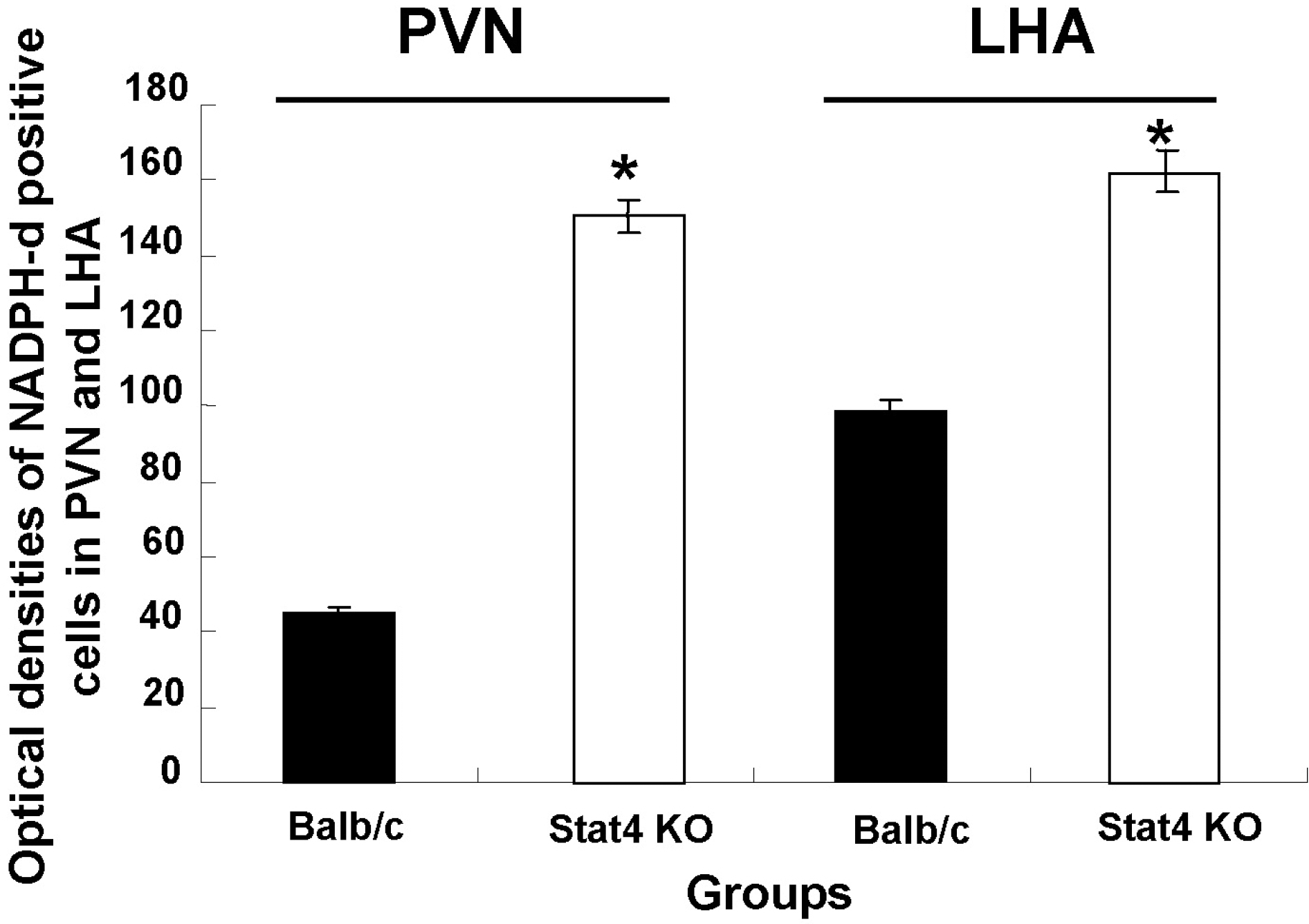
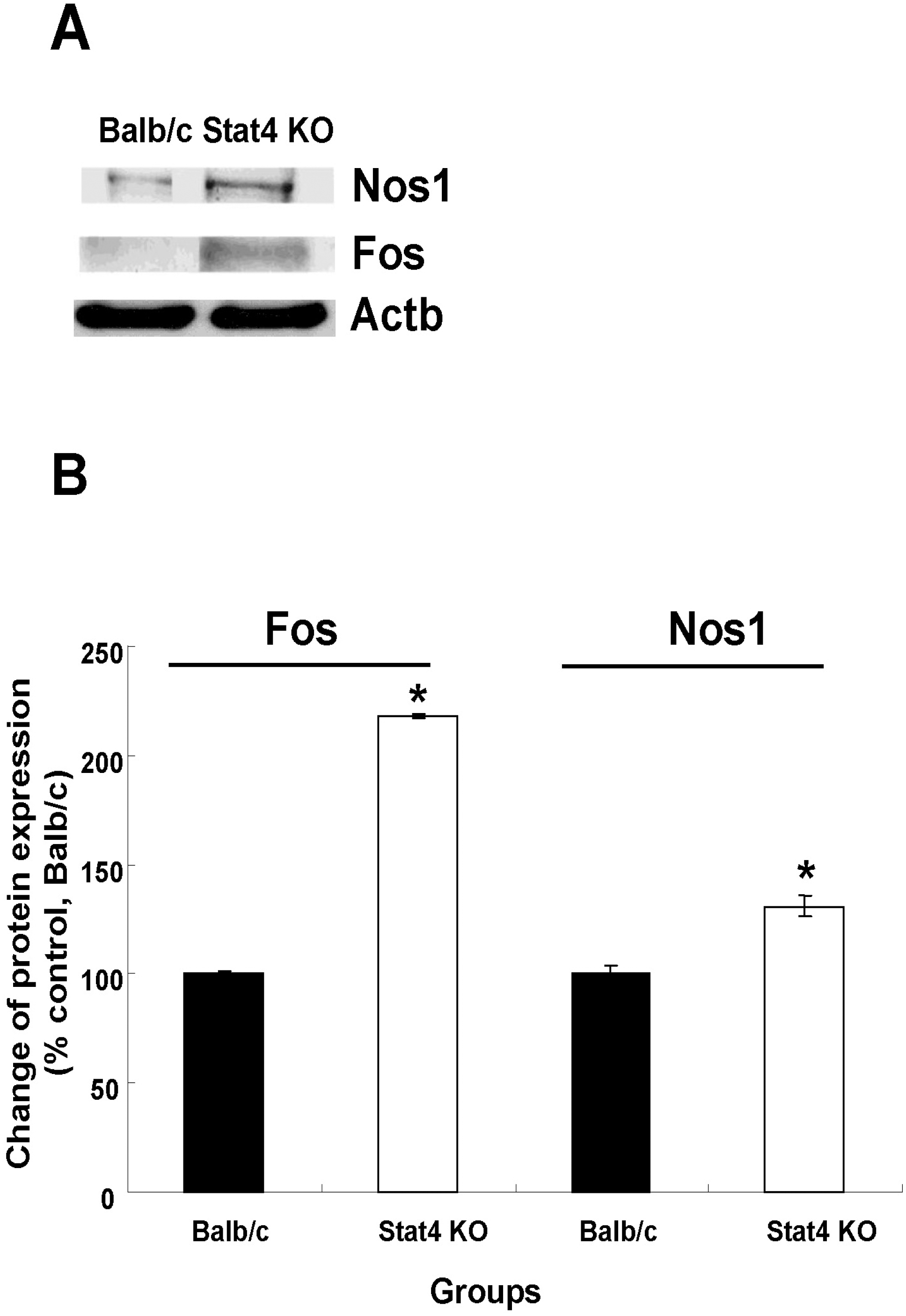
Fig. 5.
Western blotting analysis of Nos1 and Fos immunoreactivity. (A) Immunoreactive bands on western blots. (B) Expression of Nos1 and Fos proteins was calculated by densitometer and normalized to that of Actb (internal control). Values are mean±SEM. Nos1, nitric oxide synthase 1, neuronal; Fos, FBJ osteosarcoma oncogene; Actb, actin, beta; Stat4 KO, Stat4 knockout mice. ∗p<0.05 vs. Balb/c mice.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download