Abstract
Astragalus Membranaceus (AM) is a useful Korean herb that has been clinically prescribed for stress-related illness. The objective of the present study was to examine the anti-stress effects of AM on repeated stress-induced alterations of anxiety, learning and memory in rats. Restraint stress was administered for 14 days (2h/day) and AM (400mg/kg) given by oral administration, in the AM group, for the same period. Starting on the eighth day, the rats were tested for spatial memory on the Morris water maze test (MW) and for anxiety on the elevated plus maze (EPM). Changes of expression on immunohistochemistry were studied for cholineacetyl transferase (ChAT) and tyrosine hydroxylase (TH) in the brain. The results showed that the rats treated with AM had significantly reduced stress-induced deficits on learning and memory on the spatial memory tasks. In addition, the ChAT immunoreactivities were increased. In the EPM, treatment with AM increased the time spent in the open arms (p< 0.001) compared to the control group. In addition, AM treatment also normalized increases of TH expression in the LC (p < 0.001). In conclusion, administration of AM improved spatial learning and memory and reduced stress-induced anxiety. Thus, the present results suggest that AM is able to recover behavioral and neurochemical impairments induced by stress.
Go to : 
REFERENCES
Ader R., Cohen N. Psychomeuroimmunology: conditioning and stress. Annu Rev Psychol. 44:53–85. 1993.
Bisagno V., Grillo CA., Piroli GG., Giraldo P., MaEwen B., Luine VN. Chronic stress alters amphetamine effects on behavior and synaprophysin levels in female rats. Pharmacol Biochem Bahav. 78:541–550. 2004.
Bodnoff SR., Humphreys AG., Lehman JC., Diamond DM., Rose GM., Meaney MJ. Enduring effects of chronic corticoserone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 15:61–69. 1995.
Chrousos GP. Stressors, stress, and neuroendocrinology integration of the adaptive response. The 1997 Hans Selye Memorial lecture. 851:311–335. 1998.
Coburn-Litvak PS., Tata DA., Gorby HE., McCloskey DP., Richardson G., Anderson BJ. Chronic corticosterone affects brain weight, and mitochondrial, but nor glial volume fraction in hippocampal area CA3. Neuroscience. 124:429–438. 2004.
Dinan TG., Aston-Jones G. Acute haloperidol increases impulse activity of brain noradrenergic neurons. Brain Res. 307:359–362. 1984.

File SE. The use of social interaction as a method for detecting anxiolytic activity of chlrodiazepoxide-like drugs. J Neurosci Meth. 2:219–238. 1980.
Foote SL., Berridge CW., Adams LM., Pineda JA. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog Brain Res. 88:521–532. 1991.

Gottesfeld Z., Kvetnansky R., Kopin IJ., Jacobowitz DM. Effects of repeated immobilization stress on glutamate decarboxylase and choline acetyltransferase in discrete brain regions. Brain Res. 152:374–378. 1978.
Krugers HJ., Goltstein PM., van der Linden S., Joels M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J neurosci. 23:3051–3055. 2006.

Luine V., Villegas M., Martinez C., McEwen BS. Repeated stress causes reversibal impairments of spatial memory performance. Brain Res. 639:167–170. 1994.
Ma S., Mifflin SW., Cunningham JT., Mortilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-drenal stress reactivity and Fos induction in the rat locus coerleus in response to subsequent immobilization stress. Neuroscience. 154:1639–1647. 2008.
McEwen BS., Stellar E. Stress and the individual:mechanisms leading to disease. Arch Intern Med. 153:2093–2101. 1993.
Mclay R., Klinski A. Changes in prescription habits with the introduction of generic fluoxetine. Mill Med. 173:100–104. 2008.

Melia KR., Rasmussen RZ., Terwillinger JW., Haycook EJ., Nestler RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase the rat locus coeruleus: Effects of chronic stress and drug treatment. J Neurochem. 58:494–502. 1992.
Mills S., Bone K. Principles and practice of phytotherapy. PART2: PRACTICAL CLINICAL GUIDES: Dosage Issues. Herbal Approaches to Pathological States. Herbal Approaches to System Dysfunctions. A Systematic Approach to Herbal Prescribing. 1st ed.Churchill Livingstone. Edinburgh;Scotland: p. p. 27–60. 2000.
Morris RG. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 408:975–979. 2000.

Pare WP. The effects of chronic environmental stress and stomach ulceration, adrenal function and consummatory behavior in the rat. J Psychol. 57:143–151. 1964.
Paxinos G., Watson C., Pennisi M. Bregma, lamda and the interaural midpoint in stereotaxic surgery with rats of different sex. Strain and Weight. 13:139–143. 1985.
Redmond DE Jr., Huang YH. The primate locus coerleus and effects of clonidine on opiate withdrawal. J Chin Psychiatry. 43:25–29. 1982.
Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul. 41:61–73. 2007.
Sousa N., Almeida OF., Holsboer F., Paula-Barbosa MM., Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 2:237–249. 1998.

Valentino RJ., Foote SL. Corticotropin-releasing factor distrups sensory responses of brain noradrenergic neurons. Neuroendocrinology. 45:28–36. 1987.
Valentino RJ., Foote SL., Page ME. The locus coeruleus as a site for integrating corticotrophin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 697:173–188. 1993.
Go to : 
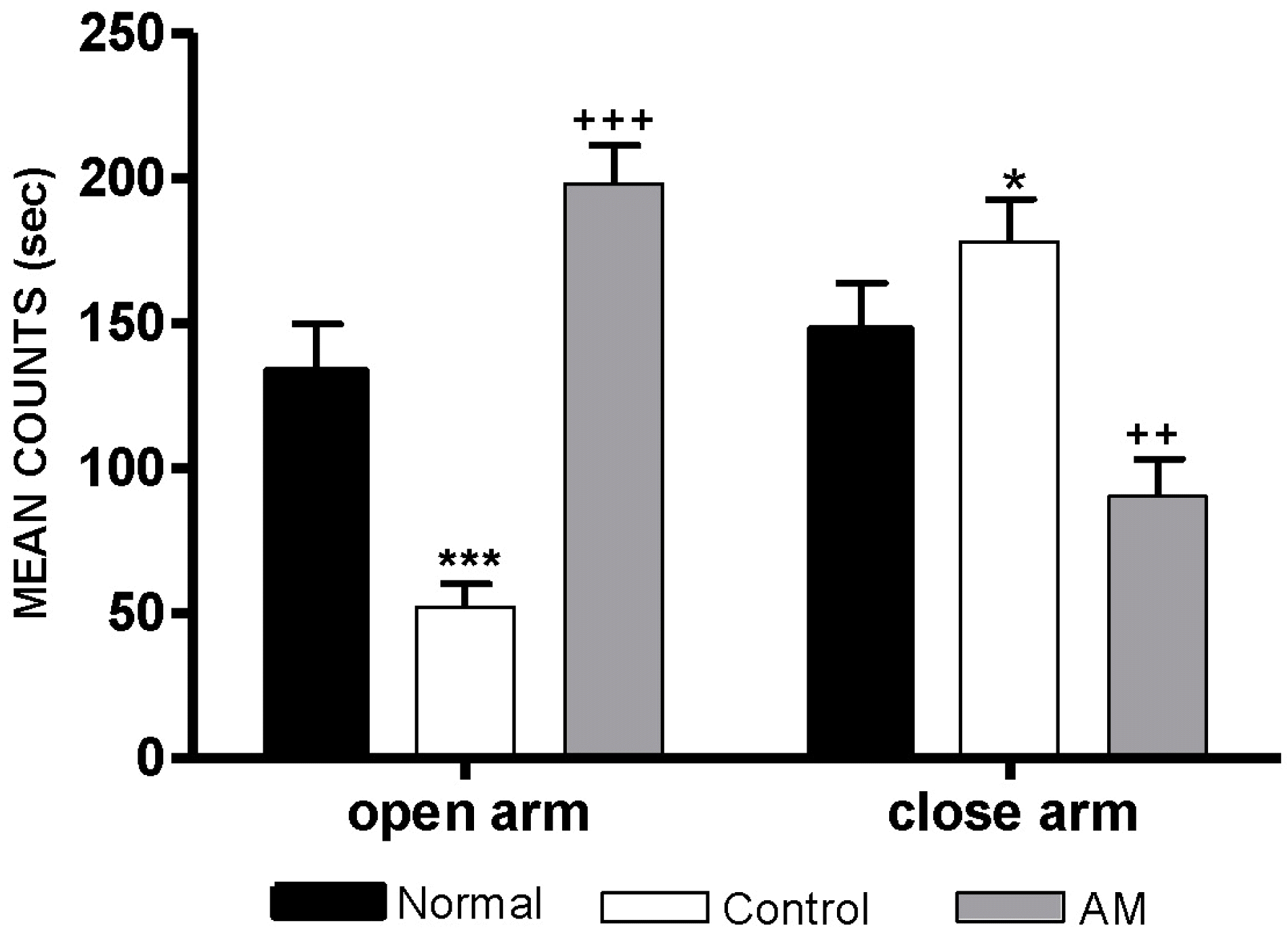 | Fig. 1.Time spent in the open and closed arms in the elevated plus maze maze. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of elevated plus maze maze were analyzed by performing separate one-way ANOVA among the groups were followed by LSD test. Each value represents the mean±S.E.M. ∗p<0.05, ∗∗∗p<0.001 compared to normal group and ++p<0.01, +++p<0.001 compared to control group, respectively. |
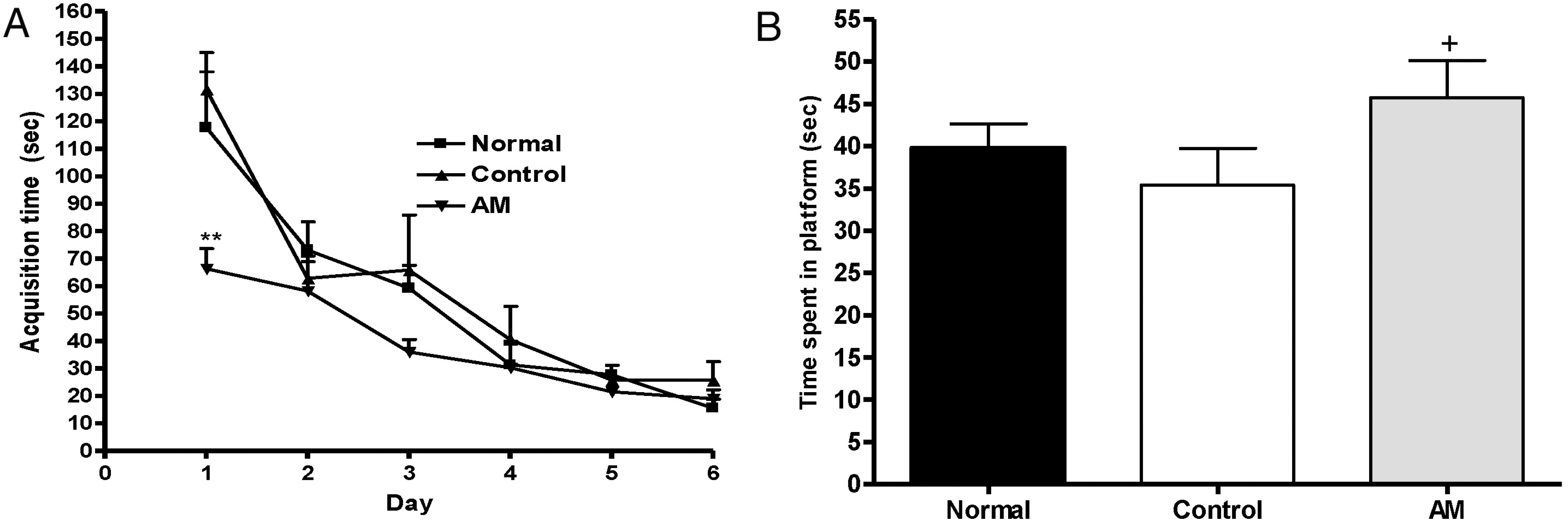 | Fig. 2.(A) Changes of the latency time during 6 d of the acquisition test in the Morris water maze test. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. Repeated measures of two-way ANOVA of swimming time among the groups following by LSD test. Each value represents the mean±S.E.M. ∗∗p<0.01 compared to normal group. (B) The latency time on the 7th day of the retention test in the Morris water maze test. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of retention test were analyzed by performing separate measures of one-way ANOVA of swimming time among the groups were followed by LSD test. Each value represents the mean±S.E.M. +p<0.05 compared to control group. |
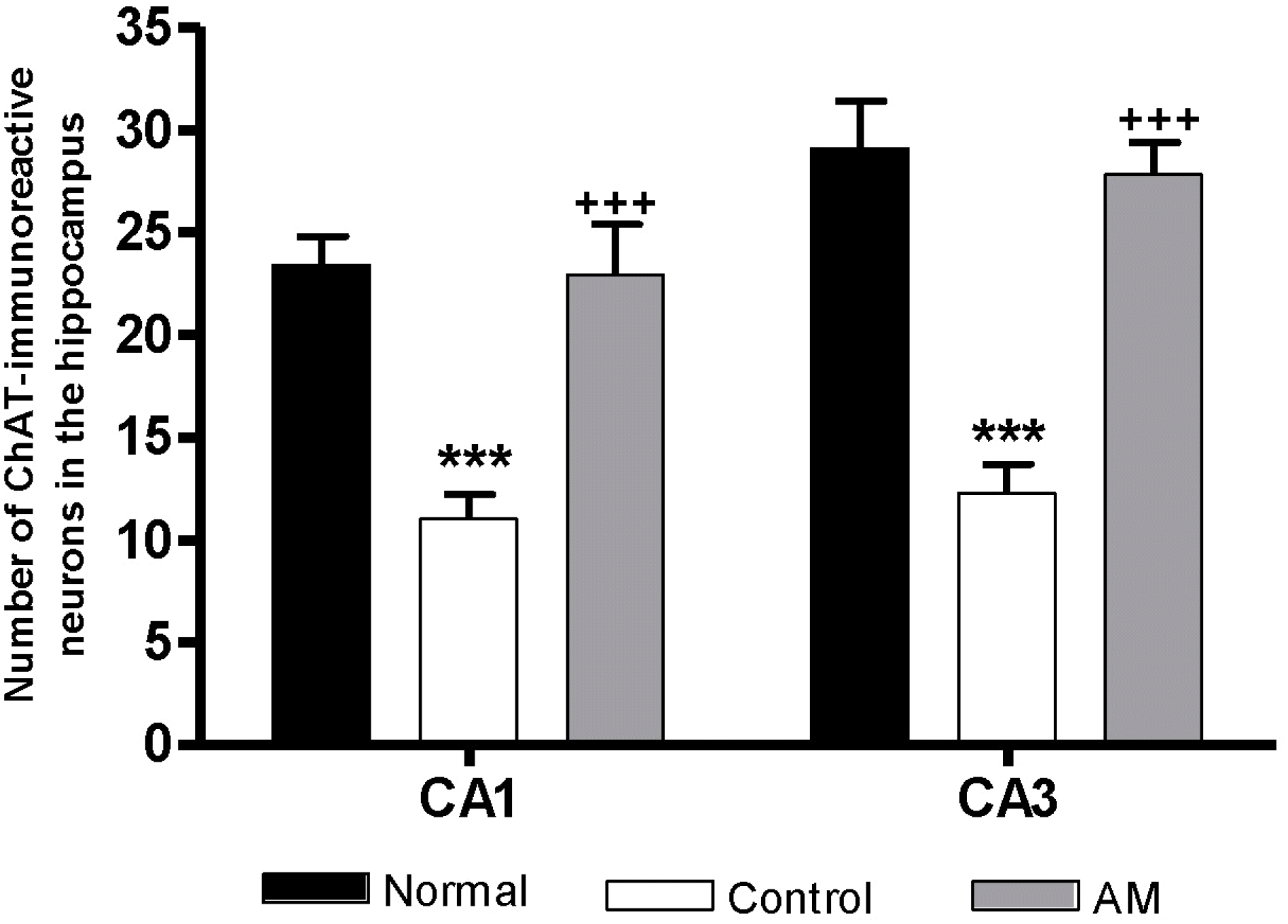 | Fig. 3.Number of choline acetyltransferase (ChAT) immunostained nuclei in the different hippocampal areas of the experimental groups after 8 d of the behavior test. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of ChAT-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups were followed by LSD test. Each value represents the mean±S.E.M. ∗∗∗p< 0.001 compared to normal group and +++p<0.001 compared to control group. |
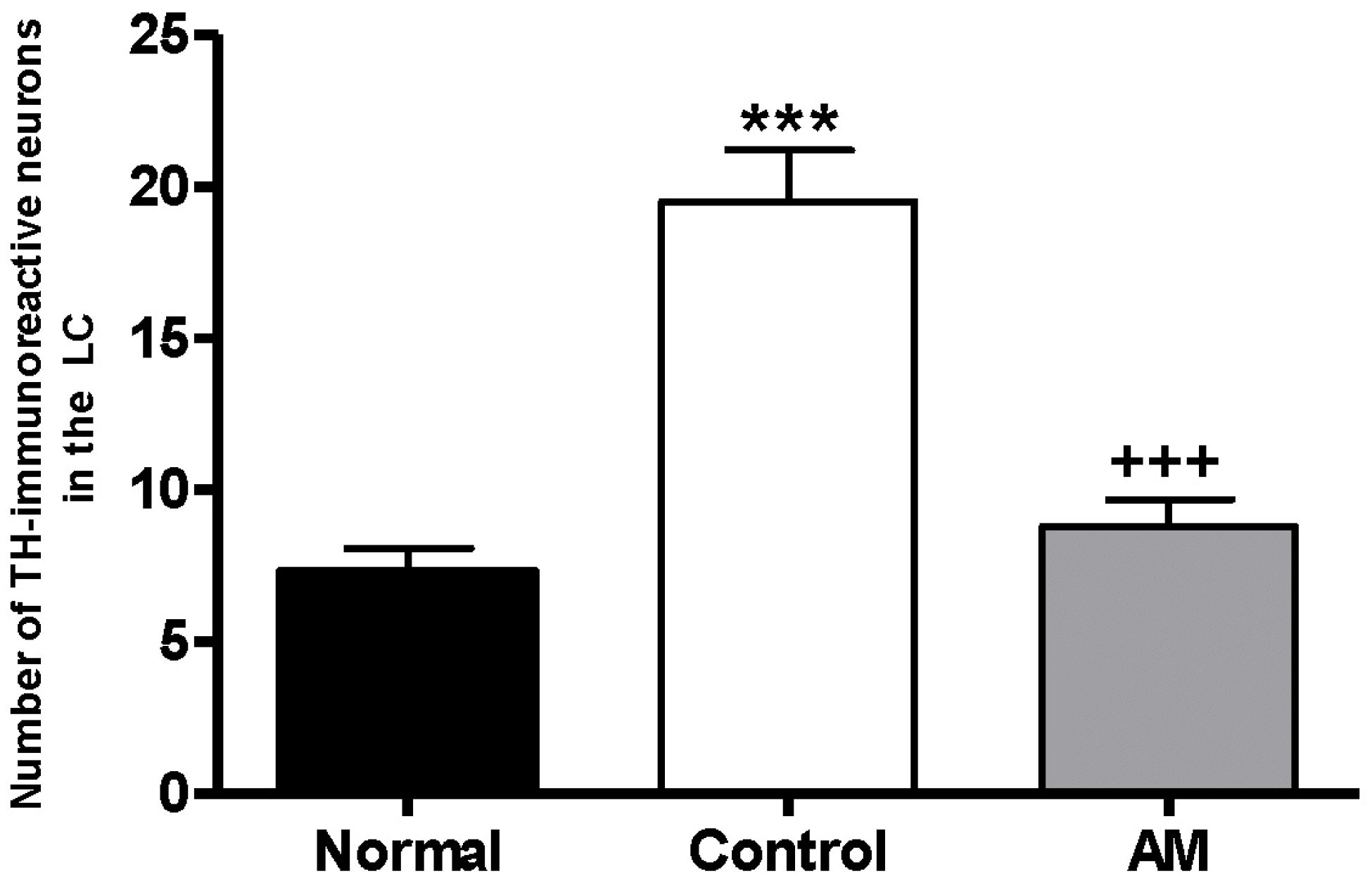 | Fig. 4.Number of Tyrosine hydroxylase (TH) immunostained nuclei in the locus coerleus areas of the experimental groups. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of TH-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups were followed by LSD test. Each value represents the mean±S.E.M. ∗∗∗p<0.001 compared to normal group and +++p<0.001 compared to control group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download