Abstract
Inflammatory processes of vascular endothelial cells play a key role in the development ofatherosclerosis. We determined the anti-inflammatory effects and mechanisms of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on LPS-treated human umbilical vein endothelial cells (HUVECs) to evaluate their cardioprotective potential. Cells were pretreated with DHA, EPA, or troglitazone prior to activation with LPS. Expression of COX-2, prostaglandin E2 (PGE2) and IL-6 production, and NF-κB activity were measured by Western blot, ELISA, and luciferase activity, respectively. Results showed that EPA, DHA, or troglitazone significantly reduced COX-2 expression, NF-κB luciferase activity, and PGE2 and IL-6 production in a dose-dependent fashion. Interestingly, low doses (10 μM) of DHA and EPA, but not troglitozone, significantly increased the activity of NF-κB in resting HUVECs. Our study suggests that while DHA, EPA, and troglitazone may be protective on HUVECs under inflammatory conditions in a dose-dependent manner. However there may be some negative effects when the concentrations are abnormally low, even in normal endothelium.
Go to : 
REFERENCES
Abeywardena MY., Head RJ. Long chain n-3 polyunsaturatedfatty acids and blood vessel function. Cardiovasc Res. 52:361–371. 2001.
Bagga D., Wang L., Farias-Eisner R., Glaspy JA., Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 100:1751–1756. 2003.
Blaschke F., Spanheimer R., Khan M., Law RE. Vascular effects of TZDs: New implications. Vascul Pharmacol. 45:3–18. 2006.

Cipollone F., Fazia ML. Cyclooxygenase-2 inhibition: vascular inflammation and cardiovascular risk. Curr Atheroscler Rep. 8:245–251. 2006.

Delerive P., Fruchart JC., Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 169:453–459. 2001.

Engler MB., Engler MM., Browne A., Sun YP., Sievers R. Mechanisms of vasorelaxation induced by eicosapentaenoic acid (20:5n-3) in WKY rat aorta. Br J Pharmacol. 131:1793–1799. 2000.

Goua M., Mulgrew S., Frank J., Rees D., Sneddon AA., Wahle KW. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: involvement of the transcription factor NF-kappaB? Prostaglandins Leukot Essent Fatty Acids. 78:33–43. 2008.
Kazemi MR., McDonald CM., Shigenaga JK., Grunfeld C., Feingold KR. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol. 25:1220–1224. 2005.

Kim HJ., Tsoy I., Park JM., Chung JI., Shin SC., Chang KC. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 580:1391–1397. 2006.
Kris-Etherton PM., Harris WS., Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 106:2747–2757. 2002.

Massaro M., Habib A., Lubrano L., Del Turco S., Lazzerini G., Bourcier T., Weksler BB., De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP (H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A. 103:15184–15189. 2006.
Massaro M., Scoditti E., Carluccio MA., De Caterina R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 79:109–115. 2008.

Philip CC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83(Suppl):1505S–1519S. 2006.
Rosen ED., Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 276:37731–37734. 2001.

Scher JU., Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009. Feb 20. [Epub ahead of print].

Serhan CN., Clish CB., Brannon J., Colgan SP., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 192:1197–1204. 2000.
Serhan CN., Hong S., Gronert K., Colgan SP., Devchand PR., Mirick G., Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 196:1025–1037. 2002.
Stary HC., Chandler AB., Glagov S., Guyton JR., Insull W Jr., Rosenfeld ME., Schaffer SA., Schwartz CJ., Wagner WD., Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Circulation. 89:2462–2478. 1994.

Stoll LL., Denning GM., Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 24:2227–2236. 2004.

Triantafilou M., Gamper FG., Lepper PM., Mouratis MA., Schumann C., Harokopakis E., Schifferle RE., Hajishengallis G., Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. 9:2030–2039. 2007.

Umetani M., Mataki C., Minegishi N., Yamamoto M., Hamakubo T., Kodama T. Function of GATA transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 21:917–922. 2001.
Go to : 
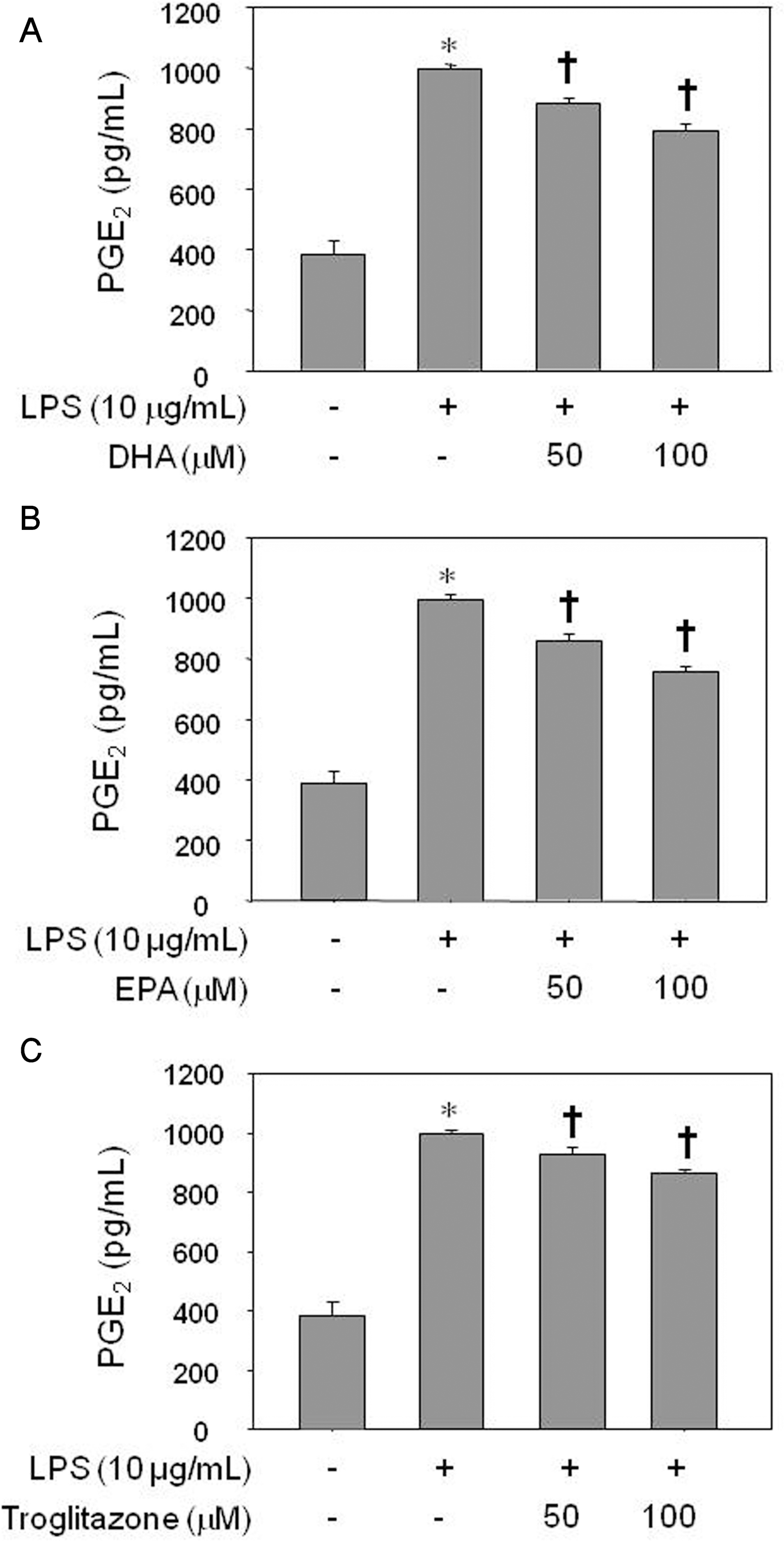 | Fig. 1.Inhibitory effect of DHA (A), EPA (B), or troglitazone (C) on PGE2 production by LPS-treated HUVECs. The results were confirmed by three experiments (n=6). Significance compared with the control, ∗p<0.05; significance compared with LPS, †p<0.05. |
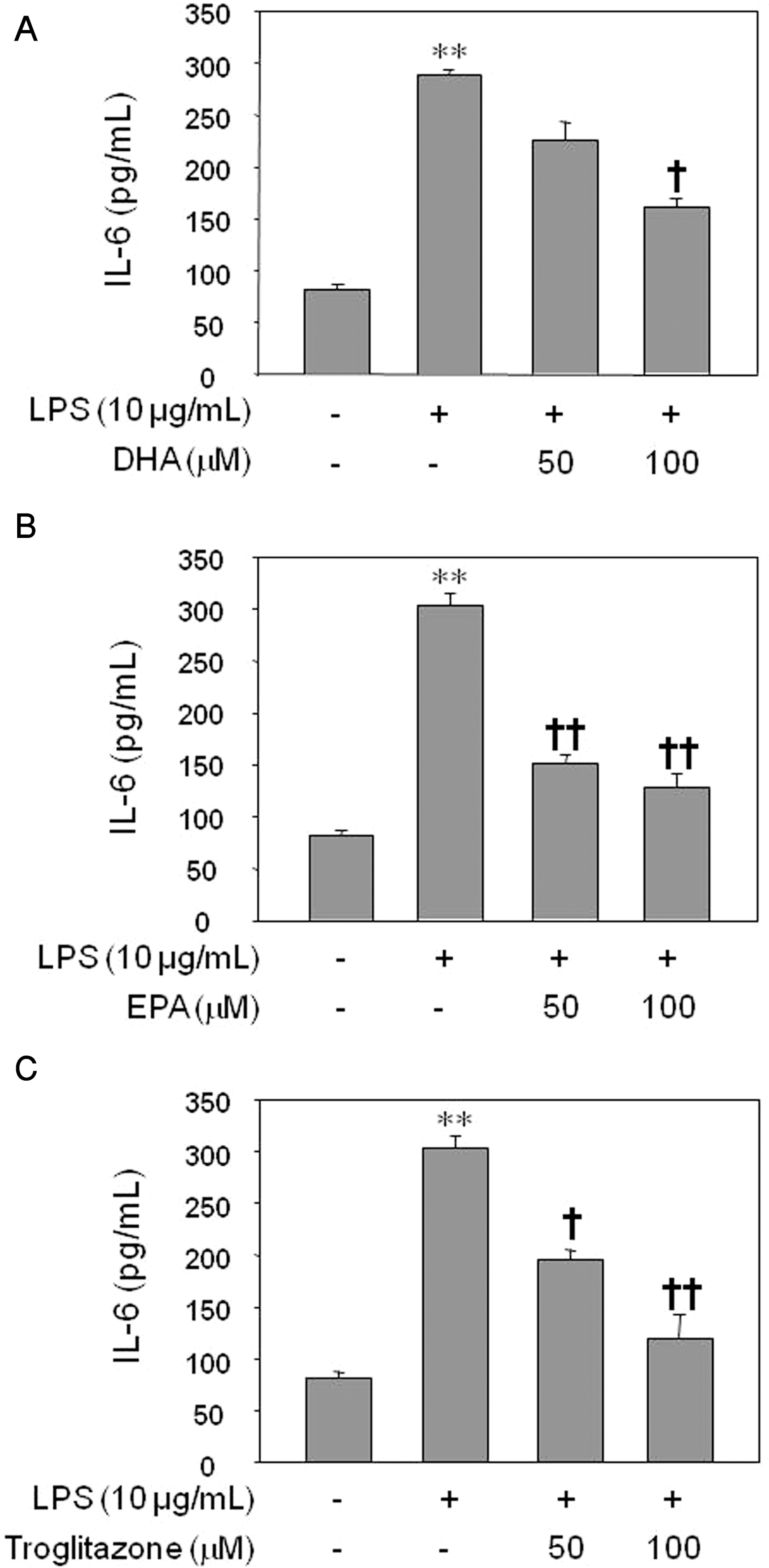 | Fig. 2.Inhibitory effect of DHA (A), EPA (B), or troglitazone (C) on IL-6 production by LPS-treated HUVECs. The results were confirmed by three experiments (n=6). Significance compared with the control, ∗∗p<0.01; significance compared with LPS, †p<0.05, ††p<0.01. |
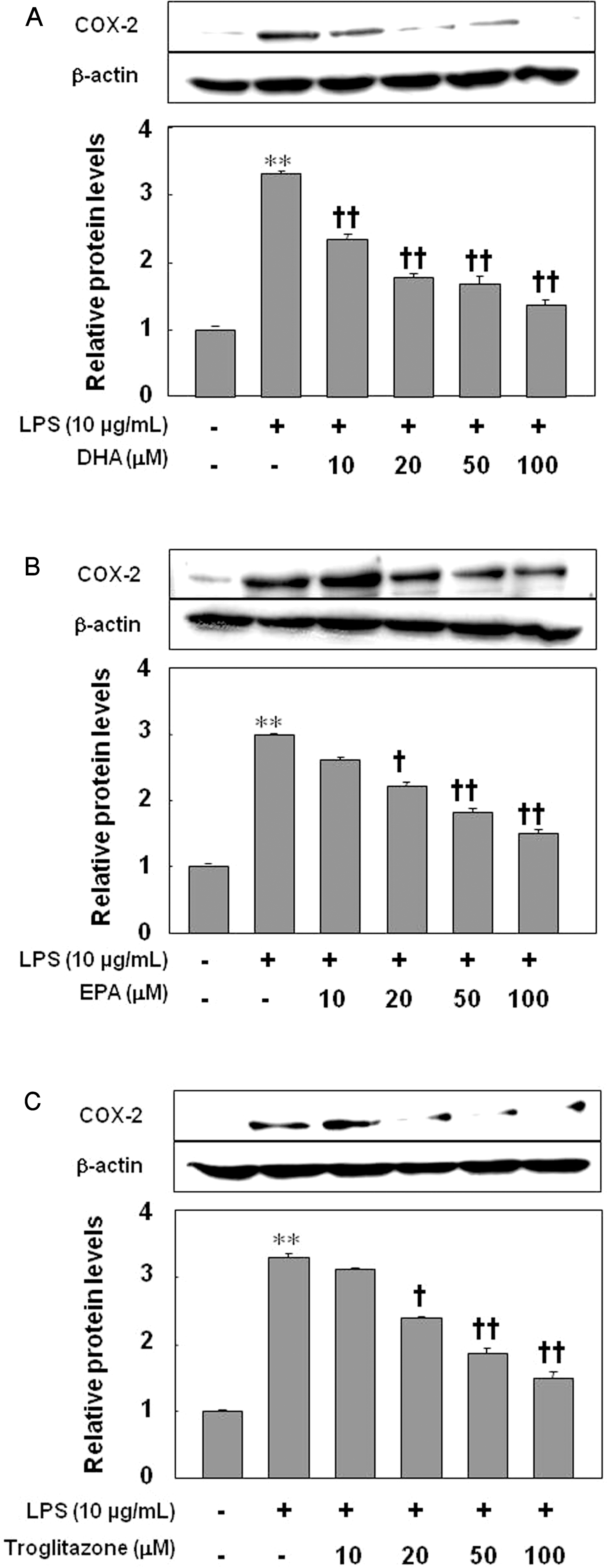 | Fig. 3.Inhibitory effect of DHA (A), EPA (B), or troglitazone (C) on the expression of COX-2. The results were confirmed by three experiments (n=3). Significance compared with the control, ∗∗p <0.01; significance compared with LPS, †p<0.05, ††p<0.01. |
 | Fig. 4.Inhibition of LPS-induced NF-κB-luciferase activity by DHA, EPA, or troglitazone. Cells were transfected with 2 μg of NF-κB-luciferase, allowed to recover for 24 h, and then stimulated with LPS in the absence of agents (A) or in the presence of DHA (B), EPA (C), or troglitazone (D). Cells were harvested 8 h after treatment, and luciferase activities were determined. The results were confirmed by three experiments (n=6). Significance compared with NF-κB transfected control, ∗p<0.05, ∗∗p<0.01; significance compared with NF-κB+LPS, †p<0.05, ††p<0.01. (E) Effect of DHA, EPA, or troglitazone on PPARγ expression by HUVECs. The results were confirmed by three experiments (n=6). Significance compared with the control, ††p<0.01. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download