Abstract
It was evaluated the inhibitory action of quercetin-3-O-β-D-glucuronopyranoside (QGC) on reflux esophagitis and gastritis in rats. QGC was isolated from the herba of Rumex Aquaticus. Reflux esophagitis or gastritis was induced surgically or by administering indomethacin, respectively. Oral QGC decreased ulcer index, injury area, gastric volume, and acid output and increased gastric pH as compared with quercetin. Furthermore, QGC significantly decreased gastric lesion sizes induced by exposing the gastric mucosa to indomethacin. Malondialdehyde levels were found to increase significantly after inducing reflux esophagitis, and were reduced by QGC, but not by quercetin or omeprazole. These results show that QGC can inhibit reflux esophagitis and gastritis in rats.
Go to : 
REFERENCES
Alvarez A., Pomar F., Sevilla ., Montero MJ. Gastric antisecretory and antiulcer activities of an ethanolic extract of Bidens pilosa L. var. radiata Schult. Bip. J Ethnopharmacol. 67:333–340. 1999.

Bell NJ., Burget D., Howden CW., Wilkinson J., Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 51(Suppl 1):59–67. 1992.

Bell NJ., Hunt RH. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut. 33:118–124. 1992.

Biancani P., Sohn UD., Rich HG., Harnett KM., Behar J. Signal transduction pathways in esophageal and lower esophageal sphincter circular muscle. Am J Med. 103:23S–28S. 1997.

Bradford MM. A rapid, sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.
Buege JA., Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 52:302–310. 1978.
Gambhir SS., Goel RK., Das Gupta G. Anti-inflammatory & anti-ulcerogenic activity of amentoflavone. Indian J Med Res. 85:689–693. 1987.
Geetha T., Malhotra V., Chopra K., Kaur IP. Antimutagenic and antioxidant/prooxidant activity of quercetin. Indian J Exp Biol. 43:61–67. 2005.
Gerritsen ME., Carley WW., Ranges GE., Shen CP., Phan SA., Ligon GF., Perry CA. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 147:278–292. 1995.
Gordon MH., Roedig-Penman A. Antioxidant activity of quercetin and myricetin in liposomes. Chem Phys Lipids. 97:79–85. 1998.

Haegele AD., Briggs SP., Thompson HJ. Antioxidant status and dietary lipid unsaturation modulate oxidative DNA damage. Free Radic Biol Med. 16:111–115. 1994.

Hollman PC., Katan MB. Bioavailability and health effects of dietary flavonols in man. Arch Toxicol Suppl. 20:237–248. 1998.

Hollman PC., van Trijp JM., Buysman MN., van der Gaag MS., Mengelers MJ., de Vries JH., Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 418:152–156. 1997.

Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 30:433–446. 2001.

Jung SY., Choi S., Ko YS., Park CS., Oh S., Koh SR., Oh U., Oh JW., Rhee MH., Nah SY. Effects of ginsenosides on vanilloid receptor (VR1) channels expressed in Xenopus oocytes. Mol Cells. 12:342–346. 2001.
Kvietys PR., Twohig B., Danzell J., Specian RD. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 98:909–920. 1990.
Lee SE., Jang HS., Song HJ., Hwang WK., Sohn UD. M1904 Downstream Signal Transduction Induced By Interleukin-1 Beta-Stimulated ROS Generation and Anti-Oxidative Effects of Quercetin-3-O-[beta]-D-Glucuronopyranoside (QGC) in Feline Esophageal Epithelial Cells. Gastroenterology. 136:A442–443. 2009.
Lewis DA. Anti-inflammatory drugs from plant, marine sources. Agents Actions Suppl. 27:3–373. 1989.
Min YS., Yim SH., Bai KL., Choi HJ., Jeong JH., Song HJ., Park SY., Ham I., Whang WK., Sohn UD. The effects of apigenin-7-O-beta-D-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton Autacoid Pharmacol. 25:85–91. 2005.

Nakamura K., Ozawa Y., Furuta Y., Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 32:445–456. 1982.

Naya MJ., Pereboom D., Ortego J., Alda JO., Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 40:175–181. 1997.

Okabe S., Takinami Y., Iwata K., Yanagawa T. Mucosal protective effect of leminoprazole on reflux esophagitis induced in rats. Jpn J Pharmacol. 69:317–323. 1995.

Parmar NS., Hennings G. The gastric antisecretory activity of 3-methoxy-5,7,3'4'-tetrahydroxyflavan (ME)–a specific histidine decarboxylase inhibitor in rats. Agents Actions. 15:143–145. 1984.

Pihan G., Regillo C., Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 32:1395–1401. 1987.

Rice-Evans CA., Miller NJ., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 20:933–956. 1996.

Sohn UD., Cho JH., Song HJ., Sun YH., Hwang WK. M1903 Protective Effects of Quercetin-3-O-[beta]-D-Glucuronopyranoside (QGC) On Ethanol-Induced Cell Damage Involve Inhibitions of ROS Generation and Downstream Activation of the ERK in Feline Esophageal Epithelial Cells. Gastroenterology. 136:A442. 2009.
Stein HJ., Hinder RA., Oosthuizen MM. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: protective role of the antioxidant glutathione. Surgery. 108:467–473. 1990.
Go to : 
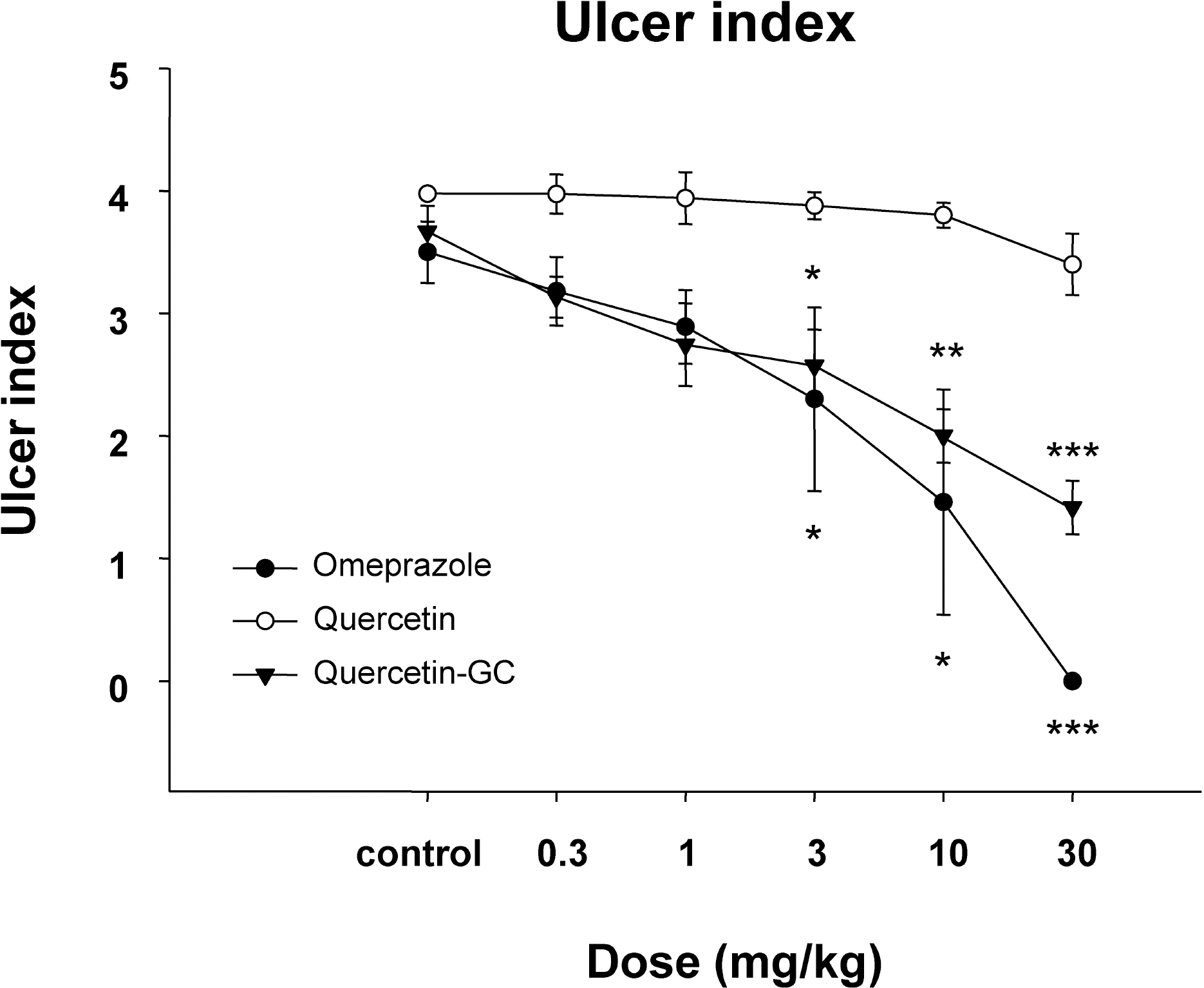 | Fig. 1.The effect of QGC on reflux esophagitis induced surgically in rats. Mean lesion area in the control group was 4 (0, no lesion; 1, ulcer area; 2, ulcer area <30 mm2; 3, ulcer area >30 mm2; 4, complete lesion (perforation of the esophageal mucosa). QGC treated animals showed significantly inhibition on reflux esophagitis. Data are means±SEMs of 6~8 animals. ∗Significantly different from the corresponding controls. ∗p<0.05, ∗∗p<0.01, and ∗∗∗p< 0.001 vs. the control. |
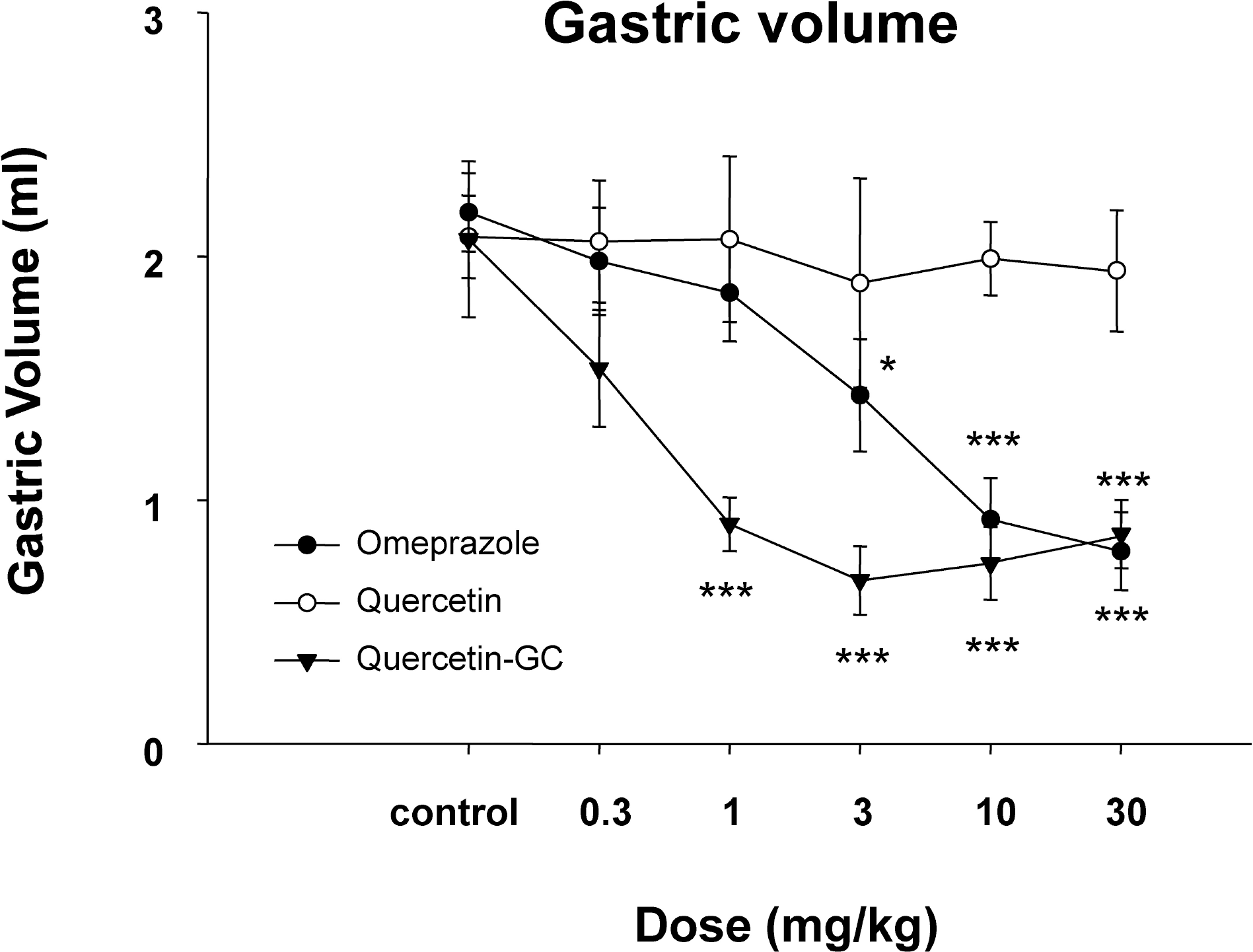 | Fig. 2.The effect of QGC on gastric volume. QGC significantly and dose-dependently decreased gastric volume. Data are means±SEM. ∗p<0.05, ∗∗p<0.01, and ∗∗∗p<0.001 vs. the control (n=6~8). |
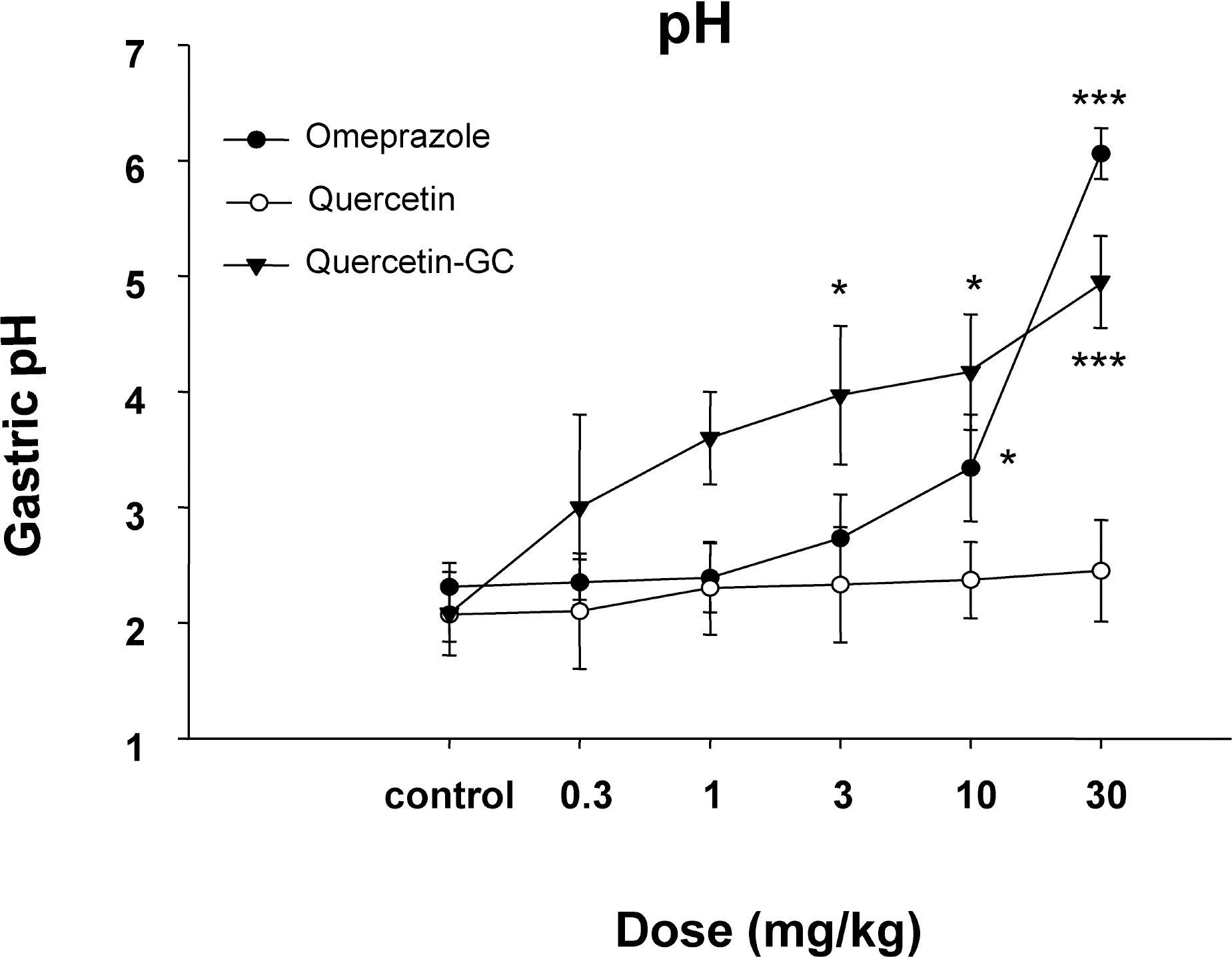 | Fig. 3.The effects of QGC on gastric pH. QGC administration changed the pH of reflux esophagitis in rats. QGC significantly increased when compared with the pH of quercetin. Data are means±SEM. ∗p<0.05, ∗∗∗p<0.001 vs. the control (n=6~8). |
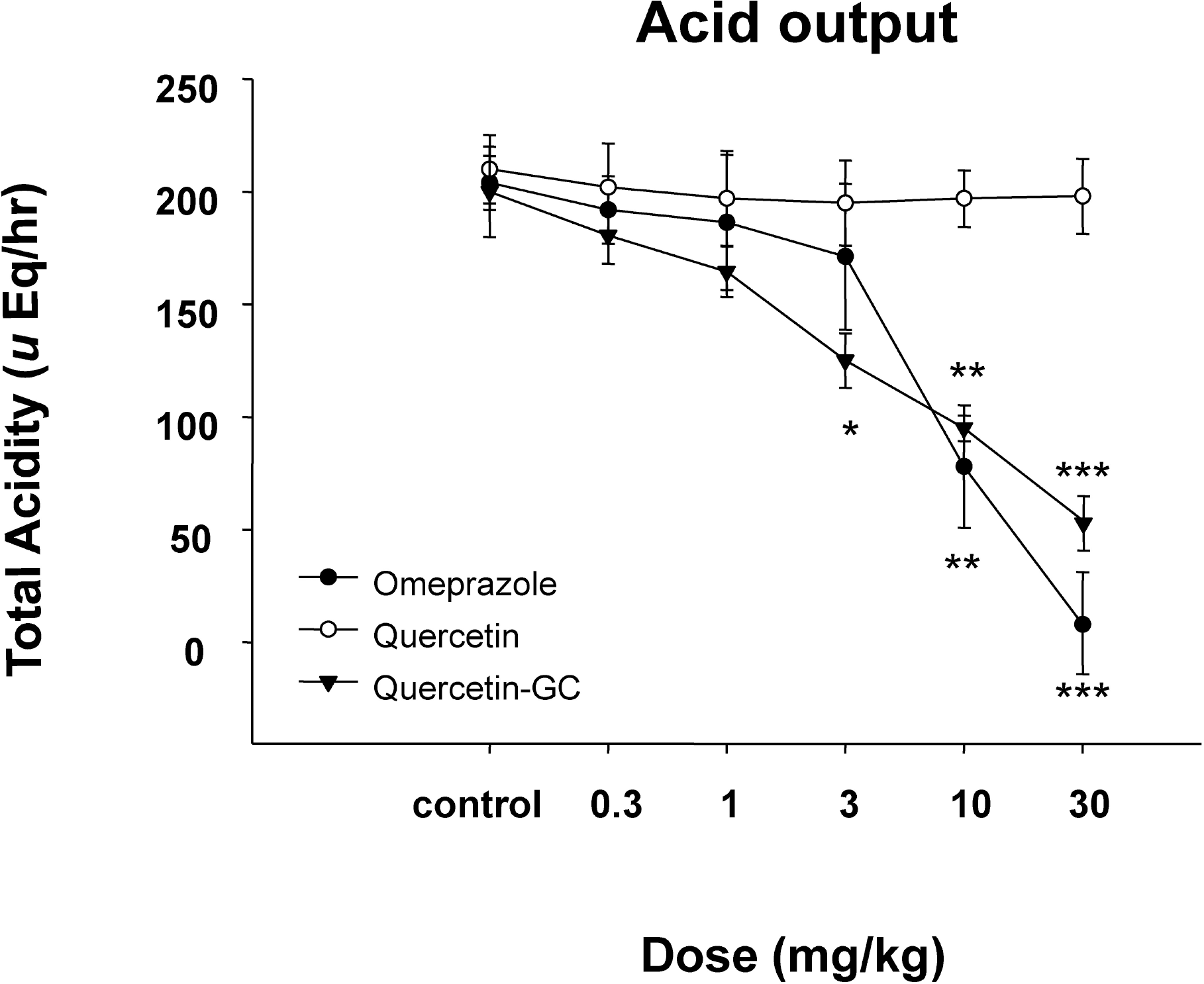 | Fig. 4.The inhibitory effect of QGC on gastric acid output. QGC significantly increased when compared to the acidity of quercetin. Data are means±SEM. ∗Significantly different from the corresponding control. ∗p<0.05, ∗∗p<0.01, and ∗∗∗p<0.001 vs. the control (n=6~8). |
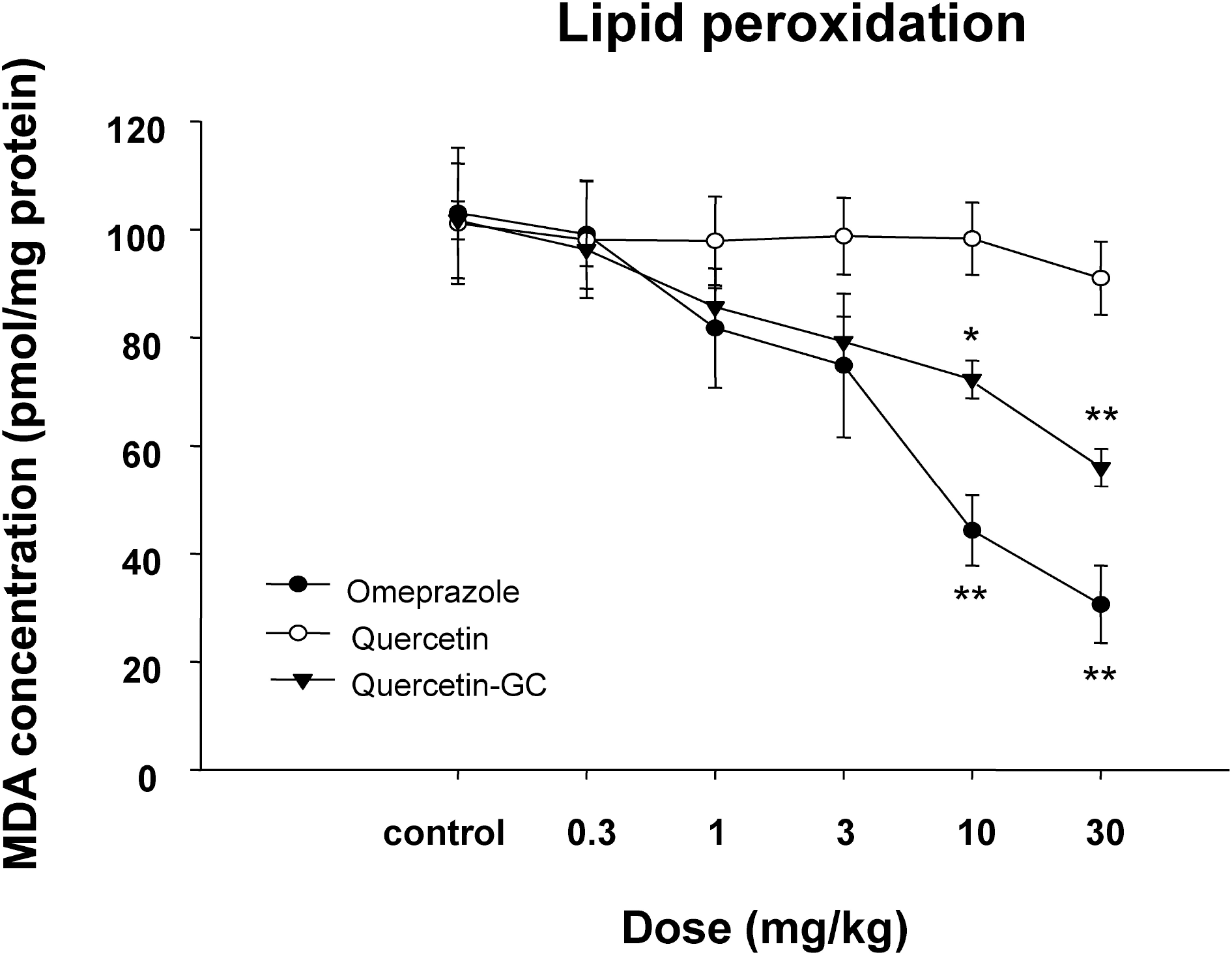 | Fig. 5.The inhibitory effects of QGC on lipid peroxidation. In the gastritis control group levels of lipid peroxidation were elevated as compared with the normal group. QGC administration significantly decreased the amount of MDA formation, suggesting that it inhibited gastritis induced lipid peroxidation. Column represent means±SEMs of 6~8 animals. ∗p<0.05 and ∗∗p<0.01 vs. control. |
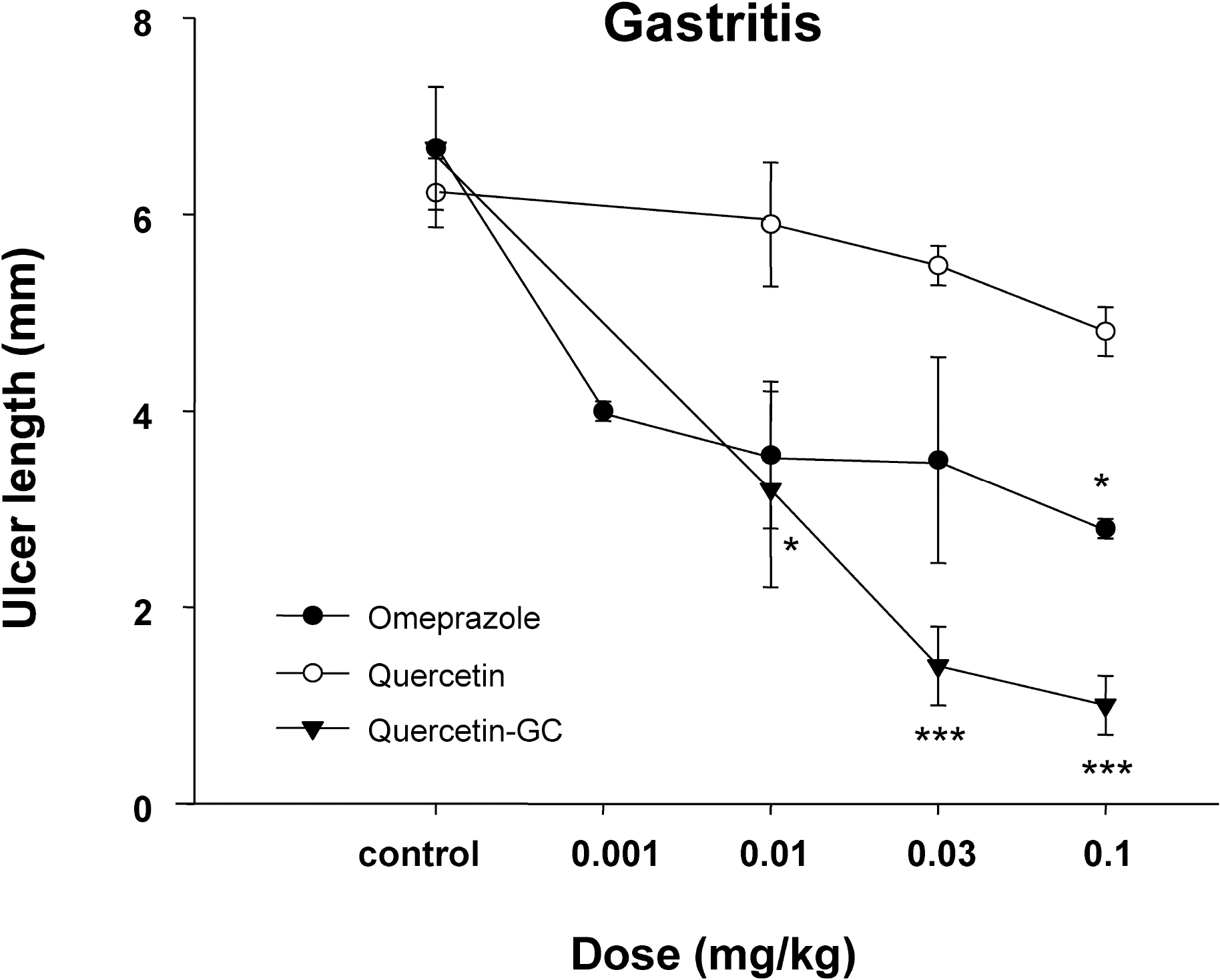 | Fig. 6.The inhibitory effect of QGC on indomethacin-induced gastritis. Mean lesion area in the control group was 4.0±0.7 cm2. QGC (0.001~0.01 mg/kg, p.o.) dose-dependently inhibited the formation of indomethacin-induced lesions. Data are means±SEM of 6~8 animals. ∗Significantly different from the corresponding control. ∗p<0.05 and ∗∗∗p<0.001 vs control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download